The Cytosolic Acetoacetyl-CoA Thiolase TaAACT1 Is Required for Defense against Fusarium pseudograminearum in Wheat
- PMID: 37047146
- PMCID: PMC10094598
- DOI: 10.3390/ijms24076165
The Cytosolic Acetoacetyl-CoA Thiolase TaAACT1 Is Required for Defense against Fusarium pseudograminearum in Wheat
Abstract
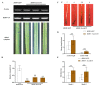
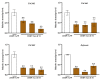
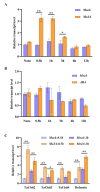
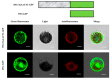
Fusarium pseudograminearum is a major pathogen for the destructive disease Fusarium crown rot (FCR) of wheat (Triticum aestivum). The cytosolic Acetoacetyl-CoA thiolase II (AACT) is the first catalytic enzyme in the mevalonate pathway that biosynthesizes isoprenoids in plants. However, there has been no investigation of wheat cytosolic AACT genes in defense against pathogens including Fusarium pseudograminearum. Herein, we identified a cytosolic AACT-encoding gene from wheat, named TaAACT1, and demonstrated its positively regulatory role in the wheat defense response to F. pseudograminearum. One haplotype of TaAACT1 in analyzed wheat genotypes was associated with wheat resistance to FCR. The TaAACT1 transcript level was elevated after F. pseudograminearum infection, and was higher in FCR-resistant wheat genotypes than in susceptible wheat genotypes. Functional analysis indicated that knock down of TaAACT1 impaired resistance against F. pseudograminearum and reduced the expression of downstream defense genes in wheat. TaAACT1 protein was verified to localize in the cytosol of wheat cells. TaAACT1 and its modulated defense genes were rapidly responsive to exogenous jasmonate treatment. Collectively, TaAACT1 contributes to resistance to F. pseudograminearum through upregulating the expression of defense genes in wheat. This study sheds new light on the molecular mechanisms underlying wheat defense against FCR.
Keywords: Fusarium pseudograminearum; cytosolic acetoacetyl-CoA thiolase; jasmonate; plant defense; wheat (Triticum aestivum).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
The Receptor-like Kinase TaCRK-7A Inhibits Fusarium pseudograminearum Growth and Mediates Resistance to Fusarium Crown Rot in Wheat.Biology (Basel). 2021 Nov 1;10(11):1122. doi: 10.3390/biology10111122. Biology (Basel). 2021. PMID: 34827115 Free PMC article.
-
A Novel Wall-Associated Kinase TaWAK-5D600 Positively Participates in Defense against Sharp Eyespot and Fusarium Crown Rot in Wheat.Int J Mol Sci. 2023 Mar 6;24(5):5060. doi: 10.3390/ijms24055060. Int J Mol Sci. 2023. PMID: 36902488 Free PMC article.
-
The Fusarium crown rot pathogen Fusarium pseudograminearum triggers a suite of transcriptional and metabolic changes in bread wheat (Triticum aestivum L.).Ann Bot. 2017 Mar 1;119(5):853-867. doi: 10.1093/aob/mcw207. Ann Bot. 2017. PMID: 27941094 Free PMC article.
-
The Wheat Wall-Associated Receptor-Like Kinase TaWAK-6D Mediates Broad Resistance to Two Fungal Pathogens Fusarium pseudograminearum and Rhizoctonia cerealis.Front Plant Sci. 2021 Oct 27;12:758196. doi: 10.3389/fpls.2021.758196. eCollection 2021. Front Plant Sci. 2021. PMID: 34777437 Free PMC article.
-
TMT-based quantitative proteomic analysis reveals defense mechanism of wheat against the crown rot pathogen Fusarium pseudograminearum.BMC Plant Biol. 2021 Feb 8;21(1):82. doi: 10.1186/s12870-021-02853-6. BMC Plant Biol. 2021. PMID: 33557748 Free PMC article.
Cited by
-
Genome-wide linkage mapping of Fusarium crown rot in common wheat (Triticum aestivum L.).Front Plant Sci. 2024 Nov 1;15:1457437. doi: 10.3389/fpls.2024.1457437. eCollection 2024. Front Plant Sci. 2024. PMID: 39554517 Free PMC article.
-
Crown rot in wheat: pathogen biology, host responses, and management strategies.Stress Biol. 2025 Aug 25;5(1):52. doi: 10.1007/s44154-025-00247-4. Stress Biol. 2025. PMID: 40851052 Free PMC article. Review.
References
-
- Zhong Z.H., Norvienyeku J., Yu J., Chen M.L., Cai R.L., Hong Y.H., Chen L.M., Zhang D.M., Wang B.H., Zhou J., et al. Two different subcellular-localized Acetoacetyl-CoA acetyltransferases differentiate diverse functions in Magnaporthe oryzae. Fungal Genet. Biol. 2015;83:58–67. doi: 10.1016/j.fgb.2015.08.008. - DOI - PubMed
-
- Wilding E.I., Brown J.R., Bryant A.P., Chalker A.F., Holmes D.J., Ingraham K.A., Iordanescu S., So C.Y., Rosenberg M., Gwynn M.N. Identification, evolution, and essentiality of the mevalonate pathway for isopentenyl diphosphate biosynthesis in gram-positive cocci. J. Bacteriol. 2000;182:4319–4327. doi: 10.1128/JB.182.15.4319-4327.2000. - DOI - PMC - PubMed
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

