Contribution of the L-Type Amino Acid Transporter Family in the Diagnosis and Treatment of Prostate Cancer
- PMID: 37047148
- PMCID: PMC10094571
- DOI: 10.3390/ijms24076178
Contribution of the L-Type Amino Acid Transporter Family in the Diagnosis and Treatment of Prostate Cancer
Abstract
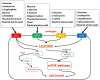
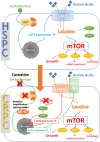
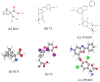
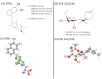
The L-type amino acid transporter (LAT) family contains four members, LAT1~4, which are important amino acid transporters. They mainly transport specific amino acids through cell membranes, provide nutrients to cells, and are involved in a variety of metabolic pathways. They regulate the mTOR signaling pathway which has been found to be strongly linked to cancer in recent years. However, in the field of prostate cancer (PCa), the LAT family is still in the nascent stage of research, and the importance of LATs in the diagnosis and treatment of prostate cancer is still unknown. Therefore, this article aims to report the role of LATs in prostate cancer and their clinical significance and application. LATs promote the progression of prostate cancer by increasing amino acid uptake, activating the mammalian target of rapamycin (mTOR) pathway and downstream signals, mediating castration-resistance, promoting tumor angiogenesis, and enhancing chemotherapy resistance. The importance of LATs as diagnostic and therapeutic targets for prostate cancer was emphasized and the latest research results were introduced. In addition, we introduced selective LAT1 inhibitors, including JPH203 and OKY034, which showed excellent inhibitory effects on the proliferation of various tumor cells. This is the future direction of amino acid transporter targeting therapy drugs.
Keywords: LAT1; LAT3; diagnosis; prostate cancer; treatment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Targeting amino acid transport in metastatic castration-resistant prostate cancer: effects on cell cycle, cell growth, and tumor development.J Natl Cancer Inst. 2013 Oct 2;105(19):1463-73. doi: 10.1093/jnci/djt241. Epub 2013 Sep 19. J Natl Cancer Inst. 2013. PMID: 24052624
-
Androgen receptor and nutrient signaling pathways coordinate the demand for increased amino acid transport during prostate cancer progression.Cancer Res. 2011 Dec 15;71(24):7525-36. doi: 10.1158/0008-5472.CAN-11-1821. Epub 2011 Oct 17. Cancer Res. 2011. PMID: 22007000
-
The LAT1 inhibitor JPH203 suppresses the growth of castration-resistant prostate cancer through a CD24-mediated mechanism.Cancer Sci. 2024 Jul;115(7):2461-2472. doi: 10.1111/cas.16191. Epub 2024 Apr 24. Cancer Sci. 2024. PMID: 38655663 Free PMC article.
-
Role of amino acid transporters in amino acid sensing.Am J Clin Nutr. 2014 Jan;99(1):223S-230S. doi: 10.3945/ajcn.113.070086. Epub 2013 Nov 27. Am J Clin Nutr. 2014. PMID: 24284439 Free PMC article. Review.
-
Molecular Mechanisms of Thyroid Hormone Transport by l-Type Amino Acid Transporter.Exp Clin Endocrinol Diabetes. 2020 Jun;128(6-07):379-382. doi: 10.1055/a-1032-8369. Epub 2019 Nov 18. Exp Clin Endocrinol Diabetes. 2020. PMID: 31739345 Review.
Cited by
-
ExpOmics: a comprehensive web platform empowering biologists with robust multi-omics data analysis capabilities.Bioinformatics. 2024 Aug 2;40(8):btae507. doi: 10.1093/bioinformatics/btae507. Bioinformatics. 2024. PMID: 39128019 Free PMC article.
-
Neurofibromin 2 modulates Mammalian Ste2-like kinases1/2 and large tumor suppressor gene1 expression in A549 lung cancer cell line.Am J Transl Res. 2024 Jun 15;16(6):2571-2578. doi: 10.62347/TPCM6776. eCollection 2024. Am J Transl Res. 2024. PMID: 39006253 Free PMC article.
-
Feasibility and Safety of Targeting Mitochondria Function and Metabolism in Acute Myeloid Leukemia.Curr Pharmacol Rep. 2024 Dec;10(6):388-404. doi: 10.1007/s40495-024-00378-8. Epub 2024 Oct 4. Curr Pharmacol Rep. 2024. PMID: 40756330 Free PMC article.
-
SLC7 transporters at the crossroads of amino acid metabolism and diabetes pathophysiology: insights and therapeutic perspectives.Front Nutr. 2025 May 21;12:1467057. doi: 10.3389/fnut.2025.1467057. eCollection 2025. Front Nutr. 2025. PMID: 40469683 Free PMC article. Review.
-
Amino acid metabolism in tumor biology and therapy.Cell Death Dis. 2024 Jan 13;15(1):42. doi: 10.1038/s41419-024-06435-w. Cell Death Dis. 2024. PMID: 38218942 Free PMC article. Review.
References
-
- Qi W., Guan Q., Sun T., Cao Y., Zhang L., Guo Y. Improving detection sensitivity of amino acids in thyroid tissues by using phthalic acid as a mobile phase additive in hydrophilic interaction chromatography-electrospray ionization-tandem mass spectrometry. Anal. Chim. Acta. 2015;870:75–82. doi: 10.1016/j.aca.2015.02.048. - DOI - PubMed
-
- Babu E., Kanai Y., Chairoungdua A., Kim D.K., Iribe Y., Tangtrongsup S., Jutabha P., Li Y., Ahmed N., Sakamoto S., et al. Identification of a Novel System L Amino Acid Transporter Structurally Distinct from Heterodimeric Amino Acid Transporters. J. Biol. Chem. 2003;278:43838–43845. doi: 10.1074/jbc.M305221200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

