Experimental Studies on the Thermal Properties and Decomposition Course of a Novel Class of Heterocyclic Anticancer Drug Candidates
- PMID: 37047158
- PMCID: PMC10094111
- DOI: 10.3390/ijms24076190
Experimental Studies on the Thermal Properties and Decomposition Course of a Novel Class of Heterocyclic Anticancer Drug Candidates
Abstract
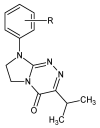
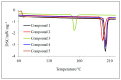
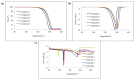
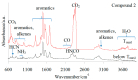
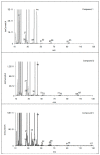
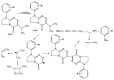
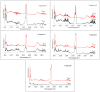
The experimental studies on the thermal properties and decomposition course of a novel class of potential anticancer drugs (1-5) containing in their heterobicyclic structures the asymmetrical triazine template were performed with the use of differential scanning calorimetry (DSC) and simultaneous thermogravimetry/differential scanning calorimetry (TG/DTG/DSC) coupled online with Fourier transform infrared spectroscopy (FTIR) and quadrupole mass spectrometry (QMS) in inert and oxidizing conditions. All the compounds were thermally characterized in detail for the first time in this article. The DSC studies proved that the melting points of the tested compounds depended on the position and type of the substituent at the phenyl moiety, whereas they did not depend on the furnace atmosphere. All the tested polynitrogenated heterocycles proved to be molecules with high thermal stability in both atmospheres, and most of them (1, 3-5) were more stable in oxidizing conditions, which indicated the formation of a more thermally stable form of the compounds when interacting with oxygen. The simultaneous TG/FTIR/QMS analyses confirmed that their pyrolysis process occurred in one main stage resulting in the emission of volatiles such as NH3, HNCO, HCN, CO, CO2, H2O, NO2, aromatic amine derivatives, alkenes (for compounds 1-5), and HCl (for the compound 5). On the other hand, the oxidative decomposition process was more complicated and proceeded in two main stages leading to the emission of NH3, CO2, CO, HCN, HNCO, H2O, some aromatics (for compounds 1-5), HCl (for compounds 3-5) as well as the additional volatiles such as N2, NO2, NH2OH, and (CN)2. The type of the formed volatiles indicated that the decomposition process of the studied heterocycles under the influence of heating was initiated by the radical mechanism. Their decomposition was related to the symmetric cleavage of C-N and C-C bonds (inert conditions) and additional reaction of the volatiles and residues with oxygen (oxidizing conditions).
Keywords: TG/DTG/DSC; TG/FTIR/QMS; annelated asymmetrical triazines; heterocyclic anticancer drug candidates; radical mechanism; thermal decomposition course; thermal stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
In Vitro, In Vivo, Ex Vivo Characterisation of Dihydroimidazotriazinones and Their Thermal Decomposition Course Studied by Coupled and Simultaneous Thermal Analysis Methods.Int J Mol Sci. 2025 Jan 10;26(2):541. doi: 10.3390/ijms26020541. Int J Mol Sci. 2025. PMID: 39859257 Free PMC article.
-
Application of Simultaneous and Coupled Thermal Analysis Techniques in Studies on the Melting Process, Course of Pyrolysis and Oxidative Decomposition of Fused Triazinylacetohydrazides.Int J Mol Sci. 2024 Jan 9;25(2):813. doi: 10.3390/ijms25020813. Int J Mol Sci. 2024. PMID: 38255887 Free PMC article.
-
Studies on the Thermal Decomposition Course of Nitrogen-Rich Heterocyclic Esters as Potential Drug Candidates and Evaluation of Their Thermal Stability and Properties.Int J Mol Sci. 2024 Apr 27;25(9):4768. doi: 10.3390/ijms25094768. Int J Mol Sci. 2024. PMID: 38731989 Free PMC article.
-
Thermal Decomposition Path-Studied by the Simultaneous Thermogravimetry Coupled with Fourier Transform Infrared Spectroscopy and Quadrupole Mass Spectrometry-Of Imidazoline/Dimethyl Succinate Hybrids and Their Biological Characterization.Materials (Basel). 2023 Jun 27;16(13):4638. doi: 10.3390/ma16134638. Materials (Basel). 2023. PMID: 37444951 Free PMC article.
-
Coupled and Simultaneous Thermal Analysis Techniques in the Study of Pharmaceuticals.Pharmaceutics. 2023 May 25;15(6):1596. doi: 10.3390/pharmaceutics15061596. Pharmaceutics. 2023. PMID: 37376045 Free PMC article. Review.
Cited by
-
In Vitro, In Vivo, Ex Vivo Characterisation of Dihydroimidazotriazinones and Their Thermal Decomposition Course Studied by Coupled and Simultaneous Thermal Analysis Methods.Int J Mol Sci. 2025 Jan 10;26(2):541. doi: 10.3390/ijms26020541. Int J Mol Sci. 2025. PMID: 39859257 Free PMC article.
References
-
- Mazur L., Łyszczek R., Sztanke M., Sztanke K. Crystal structure of 8-(4-methylphenyl)-3-(propan-2-yl)-7,8-dihydroimidazo[2,1-c][1,2,4]triazin-4(6H)-one: A potential antitumor agent. Acta Pol. Pharm. 2022;79:283–288. doi: 10.32383/appdr/150191. - DOI
-
- Sztanke M., Sztanke K., Medical University of Lublin 8-Substituted-3-(propan-2-yl)-7,8-dihydroimidazo[2,1-c][1,2,4]triazin-4(6H)-ones, Method for Obtaining Them and Medical Application. PL 236364. Polish Patent. 2021 January 11;
-
- Sztanke M., Rzymowska J., Sztanke K. Anti-Tumor Active Isopropylated Fused Azaisocytosine-Containing Congeners Are Safe for Developing Danio rerio as well as Red Blood Cells and Activate Apoptotic Caspases in Human Breast Carcinoma Cells. Molecules. 2022;27:1211. doi: 10.3390/molecules27041211. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

