Amniotic Membrane Restores Chronic Wound Features to Normal in a Keratinocyte TGF-β-Chronified Cell Model
- PMID: 37047181
- PMCID: PMC10094701
- DOI: 10.3390/ijms24076210
Amniotic Membrane Restores Chronic Wound Features to Normal in a Keratinocyte TGF-β-Chronified Cell Model
Abstract
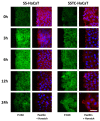
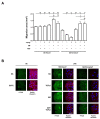
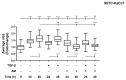
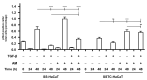
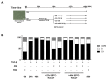
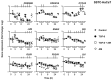
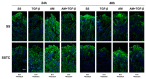
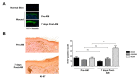
Unsuccessful wound closure in chronic wounds can be linked to altered keratinocyte activation and their inability to re-epithelize. Suggested mechanisms driving this impairment involve unbalanced cytokine signaling. However, the molecular events leading to these aberrant responses are poorly understood. Among cytokines affecting keratinocyte responses, Transforming Growth Factor-β (TFG-β) is thought to have a great impact. In this study, we have used a previously characterized skin epidermal in vitro model, HaCaT cells continuously exposed to TGF-β1, to study the wound recovery capabilities of chronified/senescent keratinocytes. In this setting, chronified keratinocytes show decreased migration and reduced activation in response to injury. Amniotic membrane (AM) has been used successfully to manage unresponsive complicated wounds. In our in vitro setting, AM treatment of chronified keratinocytes re-enabled migration in the early stages of wound healing, also promoting proliferation at later stages. Interestingly, when checking the gene expression of markers known to be altered in TGF-β chronified cells and involved in cell cycle regulation, early migratory responses, senescence, and chronic inflammation, we discovered that AM treatment seemed to reset back to keratinocyte status. The analysis of the evolution of both the levels of keratinocyte activation marker cytokeratin 17 and the spatial-temporal expression pattern of the proliferation marker Ki-67 in human in vivo biopsy samples suggests that responses to AM recorded in TGF-β chronified HaCaT cells would be homologous to those of resident keratinocytes in chronic wounds. All these results provide further evidence that sustained TGF-β might play a key role in wound chronification and postulate the validity of our TGF-β chronified HaCaT in vitro model for the study of chronic wound physiology.
Keywords: TGF-β; amniotic membrane; cell migration; cell models; cell proliferation; chronic wounds; keratinocytes; wound healing.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

