ABC Transporters in Bacterial Nanomachineries
- PMID: 37047196
- PMCID: PMC10094684
- DOI: 10.3390/ijms24076227
ABC Transporters in Bacterial Nanomachineries
Abstract
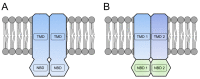
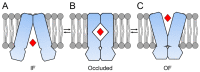
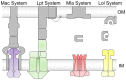
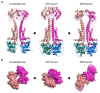
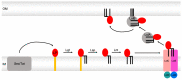
Members of the superfamily of ABC transporters are found in all domains of life. Most of these primary active transporters act as isolated entities and export or import their substrates in an ATP-dependent manner across biological membranes. However, some ABC transporters are also part of larger protein complexes, so-called nanomachineries that catalyze the vectorial transport of their substrates. Here, we will focus on four bacterial examples of such nanomachineries: the Mac system providing drug resistance, the Lpt system catalyzing vectorial LPS transport, the Mla system responsible for phospholipid transport, and the Lol system, which is required for lipoprotein transport to the outer membrane of Gram-negative bacteria. For all four systems, we tried to summarize the existing data and provide a structure-function analysis highlighting the mechanistical aspect of the coupling of ATP hydrolysis to substrate translocation.
Keywords: ABC transporter; Lol system; Lpt system; Mac system; Mla system; nanomachineries.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Combining Mutations That Inhibit Two Distinct Steps of the ATP Hydrolysis Cycle Restores Wild-Type Function in the Lipopolysaccharide Transporter and Shows that ATP Binding Triggers Transport.mBio. 2019 Aug 20;10(4):e01931-19. doi: 10.1128/mBio.01931-19. mBio. 2019. PMID: 31431556 Free PMC article.
-
Structural and functional characteristics of the tripartite ABC transporter.Microbiology (Reading). 2022 Nov;168(11). doi: 10.1099/mic.0.001257. Microbiology (Reading). 2022. PMID: 36409601 Review.
-
ATP binding and hydrolysis disrupt the high-affinity interaction between the heme ABC transporter HmuUV and its cognate substrate-binding protein.J Biol Chem. 2017 Sep 1;292(35):14617-14624. doi: 10.1074/jbc.M117.779975. Epub 2017 Jul 14. J Biol Chem. 2017. PMID: 28710276 Free PMC article.
-
Single Cell-like Systems Reveal Active Unidirectional and Light-Controlled Transport by Nanomachineries.ACS Nano. 2021 Apr 27;15(4):6747-6755. doi: 10.1021/acsnano.0c10139. Epub 2021 Mar 16. ACS Nano. 2021. PMID: 33724767 Free PMC article.
-
The Switch and Reciprocating Models for the Function of ABC Multidrug Exporters: Perspectives on Recent Research.Int J Mol Sci. 2023 Jan 30;24(3):2624. doi: 10.3390/ijms24032624. Int J Mol Sci. 2023. PMID: 36768947 Free PMC article. Review.
Cited by
-
Proteome Changes Induced by Iprodione Exposure in the Pesticide-Tolerant Pseudomonas sp. C9 Strain Isolated from a Biopurification System.Int J Mol Sci. 2024 Sep 28;25(19):10471. doi: 10.3390/ijms251910471. Int J Mol Sci. 2024. PMID: 39408799 Free PMC article.
-
Filifactor alocis enhances survival of Porphyromonas gingivalis W83 in response to H2 O2 -induced stress.Mol Oral Microbiol. 2024 Feb;39(1):12-26. doi: 10.1111/omi.12445. Epub 2023 Dec 1. Mol Oral Microbiol. 2024. PMID: 38041478 Free PMC article.
-
Genome wide structural prediction of ABC transporter systems in Bacillus subtilis.Front Microbiol. 2024 Sep 27;15:1469915. doi: 10.3389/fmicb.2024.1469915. eCollection 2024. Front Microbiol. 2024. PMID: 39397791 Free PMC article.
-
Adaptive Resistance of Staphylococcus aureus to Cefquinome Sulfate in an In Vitro Pharmacokinetic Model with Transcriptomic Insights.Microorganisms. 2025 Feb 2;13(2):329. doi: 10.3390/microorganisms13020329. Microorganisms. 2025. PMID: 40005696 Free PMC article.
-
Comparative metabolic profiling of the mycelium and fermentation broth of Penicillium restrictum from Peucedanum praeruptorum rhizosphere.Environ Microbiol Rep. 2024 Jun;16(3):e13286. doi: 10.1111/1758-2229.13286. Environ Microbiol Rep. 2024. PMID: 38844388 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

