3D Tumor Models in Urology
- PMID: 37047203
- PMCID: PMC10094462
- DOI: 10.3390/ijms24076232
3D Tumor Models in Urology
Abstract
Three-dimensional tumor models have become established in both basic and clinical research. As multicellular systems consisting of tumor and tumor-associated cells, they can better represent tumor characteristics than monocellular 2D cultures. In this review, we highlight the potential applications of tumor spheroids and organoids in the field of urology. Further, we illustrate the generation and characteristics of standardized organoids as well as membrane-based 3D in vitro models in bladder cancer research. We discuss the technical aspects and review the initial successes of molecular analyses in the three major urologic tumor entities: urinary bladder carcinoma (BCa), prostate carcinoma (PCa), and renal cell carcinoma (RCC).
Keywords: 3D cell culture technique; bladder cancer; prostate cancer; renal cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
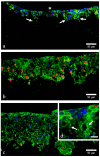

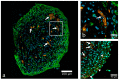

Similar articles
-
Tumor Spheroids, Tumor Organoids, Tumor Explants, and Tumoroids: What Are the Differences between Them?Biochemistry (Mosc). 2025 Feb;90(2):200-213. doi: 10.1134/S0006297924604234. Biochemistry (Mosc). 2025. PMID: 40254399 Review.
-
Three-dimensional (3D) culture of bone-derived human 786-O renal cell carcinoma retains relevant clinical characteristics of bone metastases.Cancer Lett. 2015 Aug 28;365(1):89-95. doi: 10.1016/j.canlet.2015.05.019. Epub 2015 May 21. Cancer Lett. 2015. PMID: 26004343 Free PMC article.
-
[Progress in prostate cancer study: 3D cell culture enables the ex vivo reproduction of tumor characteristics].Presse Med. 2017 Oct;46(10):954-965. doi: 10.1016/j.lpm.2017.06.014. Epub 2017 Sep 28. Presse Med. 2017. PMID: 28967525 French.
-
3D Hanging Drop Culture to Establish Prostate Cancer Organoids.Methods Mol Biol. 2017;1612:167-175. doi: 10.1007/978-1-4939-7021-6_12. Methods Mol Biol. 2017. PMID: 28634942
-
Clinical Application Perspectives of Lung Cancers 3D Tumor Microenvironment Models for In Vitro Cultures.Int J Mol Sci. 2022 Feb 18;23(4):2261. doi: 10.3390/ijms23042261. Int J Mol Sci. 2022. PMID: 35216378 Free PMC article. Review.
Cited by
-
Preclinical models for bladder cancer therapy research.Curr Opin Urol. 2024 Jul 1;34(4):244-250. doi: 10.1097/MOU.0000000000001182. Epub 2024 Apr 16. Curr Opin Urol. 2024. PMID: 38630912 Free PMC article. Review.
-
Cell Line-Based Human Bladder Organoids with Bladder-like Self-Organization-A New Standardized Approach in Bladder Cancer Research.Biomedicines. 2023 Nov 1;11(11):2958. doi: 10.3390/biomedicines11112958. Biomedicines. 2023. PMID: 38001959 Free PMC article.
-
Preclinical Models of Neuroblastoma-Current Status and Perspectives.Cancers (Basel). 2023 Jun 23;15(13):3314. doi: 10.3390/cancers15133314. Cancers (Basel). 2023. PMID: 37444423 Free PMC article. Review.
-
Human bladder organoids model urinary tract infection and bacteriophage therapy.bioRxiv [Preprint]. 2025 Jul 30:2025.07.30.667685. doi: 10.1101/2025.07.30.667685. bioRxiv. 2025. PMID: 40766562 Free PMC article. Preprint.
References
-
- Yuan Z., Zhu Z., Zhu F., Ding F., Wang Y., Wang X., Luo X., Yang J., Liu F., Sun D. Impact of human adipose tissue-derived stem cells on dermatofibrosarcoma protuberans cells in an indirect co-culture: An in vitro study. Stem Cell Res. Ther. 2021;12:1–6. doi: 10.1186/s13287-021-02512-5. - DOI - PMC - PubMed
-
- Zhang M., Xu M.X., Zhou Z., Zhang K., Zhou J., Zhao Y., Wang Z., Lu M.J. The differentiation of human adipose-derived stem cells towards a urothelium-like phenotype in vitro and the dynamic temporal changes of related cytokines by both paracrine and autocrine signal regulation. PLoS ONE. 2014;9:e95583. doi: 10.1371/journal.pone.0095583. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

