Structural Characterization of Neisseria gonorrhoeae Bacterial Peroxidase-Insights into the Catalytic Cycle of Bacterial Peroxidases
- PMID: 37047219
- PMCID: PMC10094543
- DOI: 10.3390/ijms24076246
Structural Characterization of Neisseria gonorrhoeae Bacterial Peroxidase-Insights into the Catalytic Cycle of Bacterial Peroxidases
Abstract
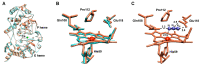
Neisseria gonorrhoeae is an obligate human pathogenic bacterium responsible for gonorrhea, a sexually transmitted disease. The bacterial peroxidase, an enzyme present in the periplasm of this bacterium, detoxifies the cells against hydrogen peroxide and constitutes one of the primary defenses against exogenous and endogenous oxidative stress in this organism. The 38 kDa heterologously produced bacterial peroxidase was crystallized in the mixed-valence state, the active state, at pH 6.0, and the crystals were soaked with azide, producing the first azide-inhibited structure of this family of enzymes. The enzyme binds exogenous ligands such as cyanide and azide, which also inhibit the catalytic activity by coordinating the P heme iron, the active site, and competing with its substrate, hydrogen peroxide. The inhibition constants were estimated to be 0.4 ± 0.1 µM and 41 ± 5 mM for cyanide and azide, respectively. Imidazole also binds and inhibits the enzyme in a more complex mechanism by binding to P and E hemes, which changes the reduction potential of the latest heme. Based on the structures now reported, the catalytic cycle of bacterial peroxidases is revisited. The inhibition studies and the crystal structure of the inhibited enzyme comprise the first platform to search and develop inhibitors that target this enzyme as a possible new strategy against N. gonorrhoeae.
Keywords: Neisseria gonorrhoeae; ROS detoxification; active state; azide-inhibited structure; bacterial peroxidase; catalytic cycle; diheme enzymes.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures








Similar articles
-
Biochemical characterization of the bacterial peroxidase from the human pathogen Neisseria gonorrhoeae.J Inorg Biochem. 2017 Jun;171:108-119. doi: 10.1016/j.jinorgbio.2017.03.007. Epub 2017 Mar 27. J Inorg Biochem. 2017. PMID: 28388513
-
YhjA - An Escherichia coli trihemic enzyme with quinol peroxidase activity.Biochim Biophys Acta Bioenerg. 2018 Jun;1859(6):411-422. doi: 10.1016/j.bbabio.2018.03.008. Epub 2018 Mar 14. Biochim Biophys Acta Bioenerg. 2018. PMID: 29550214
-
Further studies on the inactivation by sodium azide of lignin peroxidase from Phanerochaete chrysosporium.Arch Biochem Biophys. 1997 Mar 1;339(1):200-9. doi: 10.1006/abbi.1996.9839. Arch Biochem Biophys. 1997. PMID: 9056250
-
Reduction of hydrogen peroxide in gram-negative bacteria - bacterial peroxidases.Adv Microb Physiol. 2019;74:415-464. doi: 10.1016/bs.ampbs.2019.02.006. Epub 2019 Apr 8. Adv Microb Physiol. 2019. PMID: 31126534 Review.
-
Hydrogen peroxide-mediated inactivation of two chloroplastic peroxidases, ascorbate peroxidase and 2-cys peroxiredoxin.Photochem Photobiol. 2008 Nov-Dec;84(6):1404-9. doi: 10.1111/j.1751-1097.2008.00452.x. Photochem Photobiol. 2008. PMID: 19067962 Review.
Cited by
-
Coordination of the N-Terminal Heme in the Non-Classical Peroxidase from Escherichia coli.Molecules. 2023 Jun 7;28(12):4598. doi: 10.3390/molecules28124598. Molecules. 2023. PMID: 37375153 Free PMC article.
-
Biochemical Characterization of the Copper Nitrite Reductase from Neisseria gonorrhoeae.Biomolecules. 2023 Aug 4;13(8):1215. doi: 10.3390/biom13081215. Biomolecules. 2023. PMID: 37627281 Free PMC article.
-
Molecular Modeling Insights into Metal-Organic Frameworks (MOFs) as a Potential Matrix for Immobilization of Lipase: An In Silico Study.Biology (Basel). 2023 Jul 26;12(8):1051. doi: 10.3390/biology12081051. Biology (Basel). 2023. PMID: 37626937 Free PMC article.
References
-
- Johnson S.R., Steiner B.M., Cruce D.D., Perkins G.H., Arko R.J. Characterization of a catalase-deficient strain of Neisseria gonorrhoeae: Evidence for the significance of catalase in the biology of N. gonorrhoeae. Infect. Immun. 1993;61:1232–1238. doi: 10.1128/iai.61.4.1232-1238.1993. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

