Sigma-2 Receptors-From Basic Biology to Therapeutic Target: A Focus on Age-Related Degenerative Diseases
- PMID: 37047224
- PMCID: PMC10093856
- DOI: 10.3390/ijms24076251
Sigma-2 Receptors-From Basic Biology to Therapeutic Target: A Focus on Age-Related Degenerative Diseases
Abstract
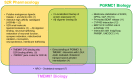
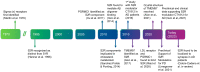
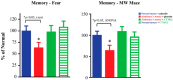
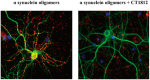
There is a large unmet medical need to develop disease-modifying treatment options for individuals with age-related degenerative diseases of the central nervous system. The sigma-2 receptor (S2R), encoded by TMEM97, is expressed in brain and retinal cells, and regulates cell functions via its co-receptor progesterone receptor membrane component 1 (PGRMC1), and through other protein-protein interactions. Studies describing functions of S2R involve the manipulation of expression or pharmacological modulation using exogenous small-molecule ligands. These studies demonstrate that S2R modulates key pathways involved in age-related diseases including autophagy, trafficking, oxidative stress, and amyloid-β and α-synuclein toxicity. Furthermore, S2R modulation can ameliorate functional deficits in cell-based and animal models of disease. This review summarizes the current evidence-based understanding of S2R biology and function, and its potential as a therapeutic target for age-related degenerative diseases of the central nervous system, including Alzheimer's disease, α-synucleinopathies, and dry age-related macular degeneration.
Keywords: Alzheimer’s disease (AD); Huntington’s disease; MAC30; Niemann–Pick disease type C (NPC); PGRMC1; Parkinson’s disease (PD); S2R; TMEM97; degenerative disease; dementia with Lewy bodies (DLB); dry age-related macular degeneration (dry AMD); prion protein, PrPC; schizophrenia; α-synuclein; σ2R.
Conflict of interest statement
M.E.H., B.N.L., M.G., A.O.C. are employees or consultants of Cognition Therapeutics Inc. S.M.C. is a former employee and founder of Cognition Therapeutics Inc. J.K. is owner of BPS International and IHS International, which have received consulting fees in the last 5 years from Cognition Therapeutics, Sage Therapeutics, Jazz Pharmaceuticals, and Greenwich Biosciences.
Figures



References
-
- Izzo N.J., Staniszewski A., To L., Fa M., Teich A.F., Saeed F., Wostein H., Walko T., Vaswani A., Wardius M., et al. Alzheimer’s Therapeutics Targeting Amyloid Beta 1–42 Oligomers I: Abeta 42 Oligomer Binding to Specific Neuronal Receptors Is Displaced by Drug Candidates That Improve Cognitive Deficits. PLoS ONE. 2014;9:e111898. doi: 10.1371/journal.pone.0111898. - DOI - PMC - PubMed
-
- Izzo N.J., Xu J., Zeng C., Kirk M.J., Mozzoni K., Silky C., Rehak C., Yurko R., Look G., Rishton G., et al. Alzheimer’s Therapeutics Targeting Amyloid Beta 1–42 Oligomers II: Sigma-2/PGRMC1 Receptors Mediate Abeta 42 Oligomer Binding and Synaptotoxicity. PLoS ONE. 2014;9:e111899. doi: 10.1371/journal.pone.0111899. - DOI - PMC - PubMed
-
- Limegrover C.S., Yurko R., Izzo N.J., LaBarbera K.M., Rehak C., Look G., Rishton G., Safferstein H., Catalano S.M. Sigma-2 Receptor Antagonists Rescue Neuronal Dysfunction Induced by Parkinson’s Patient Brain-derived A-synuclein. J. Neurosci. Res. 2021;99:1161–1176. doi: 10.1002/jnr.24782. - DOI - PMC - PubMed
-
- Jin J., Arbez N., Sahn J.J., Lu Y., Linkens K.T., Hodges T.R., Tang A., Wiseman R., Martin S.F., Ross C.A. Neuroprotective Effects of σ 2 R/TMEM97 Receptor Modulators in the Neuronal Model of Huntington’s Disease. ACS Chem. Neurosci. 2022;13:2852–2862. doi: 10.1021/acschemneuro.2c00274. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

