Plumbagin Exhibits Genotoxicity and Induces G2/M Cell Cycle Arrest via ROS-Mediated Oxidative Stress and Activation of ATM-p53 Signaling Pathway in Hepatocellular Cells
- PMID: 37047251
- PMCID: PMC10094147
- DOI: 10.3390/ijms24076279
Plumbagin Exhibits Genotoxicity and Induces G2/M Cell Cycle Arrest via ROS-Mediated Oxidative Stress and Activation of ATM-p53 Signaling Pathway in Hepatocellular Cells
Abstract
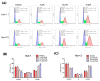
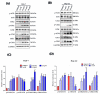
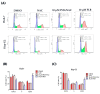
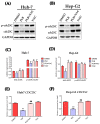
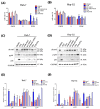
Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone, PLB), a naturally occurring naphthoquinone mainly isolated from the plant Plumbago zeylanica L., has been proven to possess anticancer activities towards multiple types of cancer. Although there has been an increasing amount of research regarding its anticancer effects, the association between oxidative stress, genotoxicity and the cell cycle arrest induced by PLB still remains unclear. Therefore, it is important to investigate their potential connections and the involvement of DNA damage and the ataxia telangiectasia mutated protein (ATM)-p53 signaling pathway in PLB's anticancer mechanism. The present study showed that PLB exposure significantly reduced HCC cell viability and colony formation. In addition, PLB-induced G2/M cell cycle arrest, oxidative stress, and DNA damage was detected, which could be almost blocked by NAC pretreatment. PLB could trigger a DNA damage response by activating cell cycle checkpoints such as ATM, checkpoint kinase 1 (Chk1), checkpoint kinase 2 (Chk2) and p53. Meanwhile, the key modulator of the G2/M transition factor, Cell Division Cycle 25C (cdc25C), was significantly downregulated in an ROS-dependent manner. Furthermore, pretreatment with ATM and p53 inhibitors (KU55933 and Pifithrin-α) could reduce the occurrence of G2/M cell cycle arrest by inhibiting the activation of the ATM-p53 pathway. Taken together, these results indicate that ROS-mediated oxidative stress plays a key role in PLB-induced G2/M cell cycle arrest mediated by the ATM-p53 pathway.
Keywords: ATM/p53 pathway; DNA damage; ROS; cell cycle arrest; plumbagin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Phenethyl isothiocyanate induces DNA damage-associated G2/M arrest and subsequent apoptosis in oral cancer cells with varying p53 mutations.Free Radic Biol Med. 2014 Sep;74:1-13. doi: 10.1016/j.freeradbiomed.2014.06.008. Epub 2014 Jun 19. Free Radic Biol Med. 2014. PMID: 24952138
-
Jaridonin-induced G2/M phase arrest in human esophageal cancer cells is caused by reactive oxygen species-dependent Cdc2-tyr15 phosphorylation via ATM-Chk1/2-Cdc25C pathway.Toxicol Appl Pharmacol. 2015 Jan 15;282(2):227-36. doi: 10.1016/j.taap.2014.11.003. Epub 2014 Nov 20. Toxicol Appl Pharmacol. 2015. PMID: 25450480 Free PMC article.
-
Cucurbitacin B induced ATM-mediated DNA damage causes G2/M cell cycle arrest in a ROS-dependent manner.PLoS One. 2014 Feb 4;9(2):e88140. doi: 10.1371/journal.pone.0088140. eCollection 2014. PLoS One. 2014. PMID: 24505404 Free PMC article.
-
DNA damage checkpoint kinases in cancer.Expert Rev Mol Med. 2020 Jun 8;22:e2. doi: 10.1017/erm.2020.3. Expert Rev Mol Med. 2020. PMID: 32508294 Review.
-
Anticancer Effects and Mechanisms of Action of Plumbagin: Review of Research Advances.Biomed Res Int. 2020 Dec 1;2020:6940953. doi: 10.1155/2020/6940953. eCollection 2020. Biomed Res Int. 2020. PMID: 33344645 Free PMC article. Review.
Cited by
-
Pharmacological Features and Therapeutic Implications of Plumbagin in Cancer and Metabolic Disorders: A Narrative Review.Nutrients. 2024 Sep 8;16(17):3033. doi: 10.3390/nu16173033. Nutrients. 2024. PMID: 39275349 Free PMC article. Review.
-
The phytochemical plumbagin: mechanism behind its "pleiotropic" nature and potential as an anticancer treatment.Arch Toxicol. 2024 Nov;98(11):3585-3601. doi: 10.1007/s00204-024-03861-9. Epub 2024 Sep 13. Arch Toxicol. 2024. PMID: 39271481 Review.
-
An integrated in vitro carcinogenicity test that distinguishes between genotoxic carcinogens, non-genotoxic carcinogens, and non-carcinogens.Mutagenesis. 2024 Mar 12;39(2):69-77. doi: 10.1093/mutage/geae004. Mutagenesis. 2024. PMID: 38301659 Free PMC article. Review.
-
Advancements in Utilizing Natural Compounds for Modulating Autophagy in Liver Cancer: Molecular Mechanisms and Therapeutic Targets.Cells. 2024 Jul 12;13(14):1186. doi: 10.3390/cells13141186. Cells. 2024. PMID: 39056768 Free PMC article. Review.
-
Plumbagin Regulates Snail to Inhibit Hepatocellular Carcinoma Epithelial-Mesenchymal Transition in vivo and in vitro.J Hepatocell Carcinoma. 2024 Mar 19;11:565-580. doi: 10.2147/JHC.S452924. eCollection 2024. J Hepatocell Carcinoma. 2024. PMID: 38525157 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 2019GXNSFBA245041/Natural Science Foundation of Guangxi Province
- AD20297013/Guangxi Science and Technology Base and Talent Project
- 2018BS050/the Research Launching Fund Project from Guangxi University of Chinese Medicine Introduced the Doctoral
- 2019GXNSFDA245003/Key projects of Guangxi Natural Science Foundation
- 2021001/The second batch of "Qihuang Project" high-level talent team cultivation project of Guangxi University of Traditional Chinese Medicine
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

