An Innovative Fluid Dynamic System to Model Inflammation in Human Skin Explants
- PMID: 37047256
- PMCID: PMC10094544
- DOI: 10.3390/ijms24076284
An Innovative Fluid Dynamic System to Model Inflammation in Human Skin Explants
Abstract
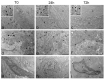
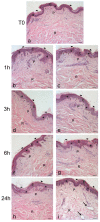
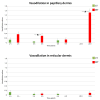
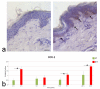
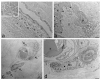
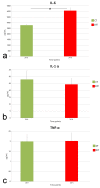
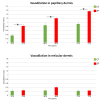
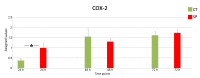
Skin is a major administration route for drugs, and all transdermal formulations must be tested for their capability to overcome the cutaneous barrier. Therefore, developing highly reliable skin models is crucial for preclinical studies. The current in vitro models are unable to replicate the living skin in all its complexity; thus, to date, excised human skin is considered the gold standard for in vitro permeation studies. However, skin explants have a limited life span. In an attempt to overcome this problem, we used an innovative bioreactor that allowed us to achieve good structural and functional preservation in vitro of explanted human skin for up to 72 h. This device was then used to set up an in vitro inflammatory model by applying two distinct agents mimicking either exogenous or endogenous stimuli: i.e., dithranol, inducing the contact dermatitis phenotype, and the substance P, mimicking neurogenic inflammation. Our in vitro system proved to reproduce inflammatory events observed in vivo, such as vasodilation, increased number of macrophages and mast cells, and increased cytokine secretion. This bioreactor-based system may therefore be suitably and reliably used to simulate in vitro human skin inflammation and may be foreseen as a promising tool to test the efficacy of drugs and cosmetics.
Keywords: bioreactor; cytokines; dithranol; in vitro tissue preservation; light microscopy; macrophages; mast cells; substance P; transmission electron microscopy; vasodilation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Integrated Microscopy and Metabolomics to Test an Innovative Fluid Dynamic System for Skin Explants In Vitro.Microsc Microanal. 2021 Aug;27(4):923-934. doi: 10.1017/S1431927621012010. Microsc Microanal. 2021. PMID: 34311807
-
Transdermal permeation of drugs with differing lipophilicity: Effect of penetration enhancer camphor.Int J Pharm. 2016 Jun 30;507(1-2):90-101. doi: 10.1016/j.ijpharm.2016.05.004. Epub 2016 May 3. Int J Pharm. 2016. PMID: 27154251
-
Recent developments in skin mimic systems to predict transdermal permeation.Curr Pharm Des. 2015;21(20):2725-32. doi: 10.2174/1381612821666150428124733. Curr Pharm Des. 2015. PMID: 25925123 Review.
-
A molecular mechanism investigation of the transdermal/topical absorption classification system on the basis of drug skin permeation and skin retention.Int J Pharm. 2021 Oct 25;608:121082. doi: 10.1016/j.ijpharm.2021.121082. Epub 2021 Sep 8. Int J Pharm. 2021. PMID: 34506925
-
Permeation enhancers in transdermal drug delivery: benefits and limitations.Expert Opin Drug Deliv. 2020 Feb;17(2):145-155. doi: 10.1080/17425247.2020.1713087. Epub 2020 Jan 14. Expert Opin Drug Deliv. 2020. PMID: 31910342 Review.
Cited by
-
Can breakthroughs in dermal and transdermal macromolecule delivery surmount existing barriers and revolutionize future therapeutics?J Transl Med. 2025 May 7;23(1):513. doi: 10.1186/s12967-025-06219-6. J Transl Med. 2025. PMID: 40336019 Free PMC article. Review.
-
Orthogonal upconversion supramolecular microneedles promote endogenous ferroptosis in keloids.Theranostics. 2025 May 8;15(13):6184-6202. doi: 10.7150/thno.108289. eCollection 2025. Theranostics. 2025. PMID: 40521195 Free PMC article.
-
Recreating Human Skin In Vitro: Should the Microbiota Be Taken into Account?Int J Mol Sci. 2024 Jan 18;25(2):1165. doi: 10.3390/ijms25021165. Int J Mol Sci. 2024. PMID: 38256238 Free PMC article. Review.
-
A Fluid Dynamic In Vitro System to Study the Effect of Hyaluronic Acid Administration on Collagen Organization in Human Skin Explants.Int J Mol Sci. 2025 Jun 4;26(11):5397. doi: 10.3390/ijms26115397. Int J Mol Sci. 2025. PMID: 40508206 Free PMC article.
References
-
- Ng K.W., Lau W.M. Skin deep: The basics of human skin structure and drug penetration. In: Dragicevic N., Maibach H.I., editors. Percutaneous Penetration Enhancers: Chemical Methods in Penetration Enhancement. Springer; Berlin/Heidelberg, Germany: 2015. pp. 3–11. - DOI
-
- Russell W.M.S., Burch R.L. The Principles of Humane Experimental Technique. 1st ed. Methuen & Co.; London, UK: 1959. pp. 1–252.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

