Biomarkers of Exposure and Potential Harm in Two Weeks of Smoking Abstinence: Changes in Biomarkers of Platelet Function, Oxidative Stress, and Inflammation
- PMID: 37047257
- PMCID: PMC10093936
- DOI: 10.3390/ijms24076286
Biomarkers of Exposure and Potential Harm in Two Weeks of Smoking Abstinence: Changes in Biomarkers of Platelet Function, Oxidative Stress, and Inflammation
Abstract
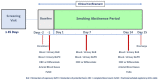
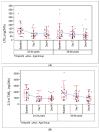
: Chronic cigarette smoking is a major risk factor for many serious diseases. While complete cessation of smoking is the best option to reduce harm from smoking, adverse impacts of smoking on health could persist for several years after cessation. Therefore, Biomarkers of Potential Harm (BoPH) are useful in interim evaluations of the beneficial effects of smoking cessation or switching to potentially lower-risk tobacco products. A 14-day smoking abstinence study was conducted under clinical confinement conditions and enrolled 70 subjects into younger (24-34 years, n = 33) and older (35-60 years, n = 37) age cohorts. Biomarkers of Exposure (BoE), which indicate exposure to nicotine and other toxicants, were measured at baseline, 7 and 14 days. Several BoPH including previously identified eicosanoids (leukotriene 4 (LTE4) and 2,3-dinor thromboxane 2 (2,3-d-TXB2) and others were evaluated. Significant declines in BoE, LTE4, 2,3-d-TXB2, neutrophils, WBC and select RBC, and arterial blood gas parameters were observed in both age cohorts at Days 7 and 14 compared to baseline, while other BoPH (e.g., FeNO) showed age-related effects. Rapid and reproducible reductions in LTE4, 2,3-d-TXB2 WBC, and neutrophil counts were consistently detected following smoking abstinence, indicating the value of these markers as useful BoPH.
Keywords: 2,3-d-TXB2; LTE4; WBC; biomarkers of exposure; biomarkers of potential harm; neutrophils; smoking abstinence.
Conflict of interest statement
All the authors are current or former employees of RAIS. The SAB study was funded by the RAI Services Company. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., which is a wholly owned subsidiary of British American Tobacco plc. P.M., E.S., P.C. and are current full-time employees of RAIS. H.-P.L., B.A.J. and G.L.P. are former employees of RAIS. G.L.P. works as an independent scientific consultant.
Figures
Similar articles
-
Urinary Leukotriene E4 and 2,3-Dinor Thromboxane B2 Are Biomarkers of Potential Harm in Short-Term Tobacco Switching Studies.Cancer Epidemiol Biomarkers Prev. 2019 Dec;28(12):2095-2105. doi: 10.1158/1055-9965.EPI-19-0342. Epub 2019 Sep 26. Cancer Epidemiol Biomarkers Prev. 2019. PMID: 31558507
-
Part three: a randomized study to assess biomarker changes in cigarette smokers switched to Vuse Solo or Abstinence.Sci Rep. 2022 Nov 30;12(1):20658. doi: 10.1038/s41598-022-25054-z. Sci Rep. 2022. PMID: 36450821 Free PMC article. Clinical Trial.
-
Effects of Switching to the Menthol Tobacco Heating System 2.2, Smoking Abstinence, or Continued Cigarette Smoking on Clinically Relevant Risk Markers: A Randomized, Controlled, Open-Label, Multicenter Study in Sequential Confinement and Ambulatory Settings (Part 2).Nicotine Tob Res. 2018 Jan 5;20(2):173-182. doi: 10.1093/ntr/ntx028. Nicotine Tob Res. 2018. PMID: 28177498 Free PMC article.
-
Evaluation of behavioural, chemical, toxicological and clinical studies of a tobacco heated product glo™ and the potential for bridging from a foundational dataset to new product iterations.Toxicol Rep. 2022 Jun 25;9:1426-1442. doi: 10.1016/j.toxrep.2022.06.014. eCollection 2022. Toxicol Rep. 2022. PMID: 36561950 Free PMC article. Review.
-
Biomarkers of Potential Harm: Summary of an FDA-Sponsored Public Workshop.Nicotine Tob Res. 2019 Jan 1;21(1):3-13. doi: 10.1093/ntr/ntx273. Nicotine Tob Res. 2019. PMID: 29253243 Review.
Cited by
-
Changes in Biomarkers of Exposure and Potential Harm in Smokers Switched to Vuse Vibe or Vuse Ciro Electronic Nicotine Delivery Systems.Toxics. 2023 Jun 29;11(7):564. doi: 10.3390/toxics11070564. Toxics. 2023. PMID: 37505530 Free PMC article.
-
Relationship between urinary tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and lung function: Evidence from NHANES 2007-2012.Tob Induc Dis. 2023 Dec 12;21:165. doi: 10.18332/tid/175009. eCollection 2023. Tob Induc Dis. 2023. PMID: 38090741 Free PMC article.
-
Trends in hemoglobin-to- red cell distribution width ratio and its prognostic value for all-cause, cancer, and cardiovascular mortality: a nationwide cohort study.Sci Rep. 2025 Mar 5;15(1):7685. doi: 10.1038/s41598-025-92228-w. Sci Rep. 2025. PMID: 40044826 Free PMC article.
-
Special Issue "Molecular Research on Platelet Activity in Health and Disease 2024".Int J Mol Sci. 2025 Apr 19;26(8):3873. doi: 10.3390/ijms26083873. Int J Mol Sci. 2025. PMID: 40332555 Free PMC article.
-
Evaluation of Morphology and Biochemical Parameters of Young Adults Using Heated Tobacco Products in Poland: A Case-Control Study.J Clin Med. 2025 Apr 16;14(8):2734. doi: 10.3390/jcm14082734. J Clin Med. 2025. PMID: 40283564 Free PMC article.
References
-
- U.S. Department of Health and Human Services . A report of the Surgeon General. Centers for Disease Control and Prevention Office on Smoking and Health; Atlanta, GA, USA: 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. A Report of the Surgeon General. - PubMed
-
- U.S. Department of Health and Human Services . In: Smoking Cessation: A Report of the Surgeon General. U.S. Department of Health and Human Services; National Center for Chronic Disease Centers for Disease Control and Prevention and Office on Smoking and Health Prevention and Health Promotion, editor. U.S. Department of Health and Human Services; Atlanta, GA, USA: 2020.
-
- U.S. Congress HHS, editor. [(accessed on 28 February 2023)];Family Smoking Prevention and Tobacco Control and Federal Retirement Reform. Public Law 2009:111–31. 2009 Available online: https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/fami....
-
- Food and Drug Administration . In: Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke. Established List. U.S. Department of Health and Human Services, editor. Volume 77. Office of the Federal Register; Washington, DC, USA: 2012. pp. 20034–20037.
-
- Institute of Medicine Committee to Assess the Science Base for Tobacco Harm Reduction . In: Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Stratton K., Shetty P., Wallace R., Bondurant S., editors. National Academies Press (US); Washington, DC, USA: 2001. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

