CsCIPK11-Regulated Metalloprotease CsFtsH5 Mediates the Cold Response of Tea Plants
- PMID: 37047263
- PMCID: PMC10094637
- DOI: 10.3390/ijms24076288
CsCIPK11-Regulated Metalloprotease CsFtsH5 Mediates the Cold Response of Tea Plants
Abstract
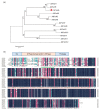
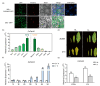
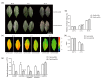
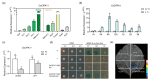
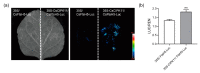
Photosystem II repair in chloroplasts is a critical process involved in maintaining a plant's photosynthetic activity under cold stress. FtsH (filamentation temperature-sensitive H) is an essential metalloprotease that is required for chloroplast photosystem II repair. However, the role of FtsH in tea plants and its regulatory mechanism under cold stress remains elusive. In this study, we cloned a FtsH homolog gene in tea plants, named CsFtsH5, and found that CsFtsH5 was located in the chloroplast and cytomembrane. RT-qPCR showed that the expression of CsFtsH5 was increased with leaf maturity and was significantly induced by light and cold stress. Transient knockdown CsFtsH5 expression in tea leaves using antisense oligonucleotides resulted in hypersensitivity to cold stress, along with higher relative electrolyte leakage and lower Fv/Fm values. To investigate the molecular mechanism underlying CsFtsH5 involvement in the cold stress, we focused on the calcineurin B-like-interacting protein kinase 11 (CsCIPK11), which had a tissue expression pattern similar to that of CsFtsH5 and was also upregulated by light and cold stress. Yeast two-hybrid and dual luciferase (Luc) complementation assays revealed that CsFtsH5 interacted with CsCIPK11. Furthermore, the Dual-Luc assay showed that CsCIPK11-CsFtsH5 interaction might enhance CsFtsH5 stability. Altogether, our study demonstrates that CsFtsH5 is associated with CsCIPK11 and plays a positive role in maintaining the photosynthetic activity of tea plants in response to low temperatures.
Keywords: CsCIPK11; CsFtsH5; cold; photosynthetic activity; tea plant.
Conflict of interest statement
All the authors in the manuscript have no conflicts of interest.
Figures
References
-
- Mishra N.P., Francke C., van Gorkom H.J., Ghanotakis D.F. Destructive role of singlet oxygen during aerobic illumi-nation of the photosystem II core complex. Biochim. Biophys. Acta. 1994;1186:81–90. doi: 10.1016/0005-2728(94)90138-4. - DOI
MeSH terms
Substances
Grants and funding
- U22A20499/National Natural Science Foundation of China
- 31870685/National Natural Science Foundation of China
- 1610212018007/Central Public Interest Scientific Institution Basal Research Fund
- 2022XTTGCY01-02/the Project for Collaborative Promotion of Major Technology of Zhejiang province
- CARS-19-01A/China Agriculture Research System of MOF and MARA
LinkOut - more resources
Full Text Sources

