Hyperglycemia Aggravates Periodontitis via Autophagy Impairment and ROS-Inflammasome-Mediated Macrophage Pyroptosis
- PMID: 37047282
- PMCID: PMC10094233
- DOI: 10.3390/ijms24076309
Hyperglycemia Aggravates Periodontitis via Autophagy Impairment and ROS-Inflammasome-Mediated Macrophage Pyroptosis
Abstract
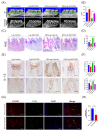
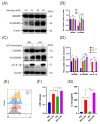
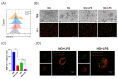
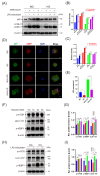
Macrophage pyroptosis drives the secretion of IL-1β, which has been recently reported to be a featured salivary biomarker for discriminating periodontitis in the presence of diabetes. This study aimed to explore whether macrophage pyroptosis plays a role in the development of diabetes mellitus-periodontitis, as well as potential therapeutic strategies. By establishing a model of experimental diabetes mellitus-periodontitis in rats, we found that IL-1β and gasdermin D were highly expressed, leading to aggravated destruction of periodontal tissue. MCC950, a potent and selective molecule inhibitor of the NLRP3 inflammasome, effectively inhibited macrophage pyroptosis and attenuated alveolar bone losses in diabetes mellitus-periodontitis. Consistently, in vitro, high glucose could induce macrophage pyroptosis and thus promoted IL-1β production in macrophages stimulated by lipopolysaccharide. In addition, autophagy blockade by high glucose via the mTOR-ULK1 pathway led to severe oxidative stress response in macrophages stimulated by lipopolysaccharide. Activation of autophagy by rapamycin, clearance of mitochondrial ROS by mitoTEMPO, and inhibition of inflammasome by MCC950 could significantly reduce macrophage pyroptosis and IL-1β secretion. Our study demonstrates that hyperglycemia promotes IL-1β production and pyroptosis in macrophages suffered by periodontal microbial stimuli. Modulation of autophagy activity and specific targeting of the ROS-inflammasome pathway may offer promising therapeutic strategies to alleviate diabetes mellitus-periodontitis.
Keywords: MCC950; autophagy; hyperglycemia; oxidative stress; periodontal disease; pyroptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Davis F.M., Tsoi L.C., Wasikowski R., denDekker A., Joshi A., Wilke C., Deng H., Wolf S., Obi A., Huang S., et al. Epigenetic regulation of the PGE2 pathway modulates macrophage phenotype in normal and pathologic wound repair. JCI Insight. 2020;5:e138443. doi: 10.1172/jci.insight.138443. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

