IL-10 Promotes CXCL13 Expression in Macrophages Following Foot-and-Mouth Disease Virus Infection
- PMID: 37047294
- PMCID: PMC10093876
- DOI: 10.3390/ijms24076322
IL-10 Promotes CXCL13 Expression in Macrophages Following Foot-and-Mouth Disease Virus Infection
Abstract
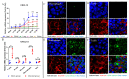
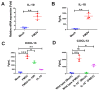
Foot-and-mouth disease (FMD) is one of the most contagious livestock diseases in the world, posing a constant global threat to the animal trade and national economies. The chemokine C-X-C motif chemokine ligand 13 (CXCL13), a biomarker for predicting disease progression in some diseases, was recently found to be increased in sera from mice infected with FMD virus (FMDV) and to be associated with the progression and severity of the disease. However, it has not yet been determined which cells are involved in producing CXCL13 and the signaling pathways controlling CXCL13 expression in these cells. In this study, the expression of CXCL13 was found in macrophages and T cells from mice infected with FMDV, and CXCL13 was produced in bone-marrow-derived macrophages (BMDMs) by activating the nuclear factor-kappaB (NF-κB) and JAK/STAT pathways following FMDV infection. Interestingly, CXCL13 concentration was decreased in sera from interleukin-10 knock out (IL-10-/-) mice or mice blocked IL-10/IL-10R signaling in vivo after FMDV infection. Furthermore, CXCL13 was also decreased in IL-10-/- BMDMs and BMDMs treated with anti-IL-10R antibody following FMDV infection in vitro. Lastly, it was demonstrated that IL-10 regulated CXCL13 expression via JAK/STAT rather than the NF-κB pathway. In conclusion, the study demonstrated for the first time that macrophages and T cells were the cellular sources of CXCL13 in mice infected with FMDV; CXCL13 was produced in BMDMs via NF-κB and JAK/STAT pathways; and IL-10 promoted CXCL13 expression in BMDMs via the JAK/STAT pathway.
Keywords: C-X-C motif chemokine ligand 13; foot-and-mouth disease virus; interleukin-10; macrophage; signaling pathways.
Conflict of interest statement
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Tsai C.H., Chen C.J., Gong C.L., Liu S.C., Chen P.C., Huang C.C., Hu S.L., Wang S.W., Tang C.H. CXCL13/CXCR5 axis facilitates endothelial progenitor cell homing and angiogenesis during rheumatoid arthritis progression. Cell Death Dis. 2021;12:846. doi: 10.1038/s41419-021-04136-2. - DOI - PMC - PubMed
-
- Litsiou E., Semitekolou M., Galani I.E., Morianos I., Tsoutsa A., Kara P., Rontogianni D., Bellenis I., Konstantinou M., Potaris K., et al. CXCL13 production in B cells via Toll-like receptor/lymphotoxin receptor signaling is involved in lymphoid neogenesis in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013;187:1194–1202. doi: 10.1164/rccm.201208-1543OC. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

