Neutrophils in Health and Disease: From Receptor Sensing to Inflammasome Activation
- PMID: 37047314
- PMCID: PMC10094305
- DOI: 10.3390/ijms24076340
Neutrophils in Health and Disease: From Receptor Sensing to Inflammasome Activation
Abstract
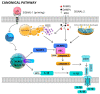
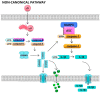
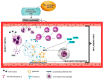
Neutrophils-polymorphonuclear cells (PMNs) are the cells of the initial immune response and make up the majority of leukocytes in the peripheral blood. After activation, these cells modify their functional status to meet the needs at the site of action or according to the agent causing injury. They receive signals from their surroundings and "plan" the course of the response in both temporal and spatial contexts. PMNs dispose of intracellular signaling pathways that allow them to perform a wide range of functions associated with the development of inflammatory processes. In addition to these cells, some protein complexes, known as inflammasomes, also have a special role in the development and maintenance of inflammation. These complexes participate in the proteolytic activation of key pro-inflammatory cytokines, such as IL-1β and IL-18. In recent years, there has been significant progress in the understanding of the structure and molecular mechanisms behind the activation of inflammasomes and their participation in the pathogenesis of numerous diseases. The available reports focus primarily on macrophages and dendritic cells. According to the literature, the activation of inflammasomes in neutrophils and the associated death type-pyroptosis-is regulated in a different manner than in other cells. The present work is a review of the latest reports concerning the course of inflammasome activation and inflammatory cytokine secretion in response to pathogens in neutrophils, as well as the role of these mechanisms in the pathogenesis of selected diseases.
Keywords: CAPS; COVID-19; cardiovascular disease; inflammasome; neutrophil; pyroptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Specific NLRP3 Inflammasome Assembling and Regulation in Neutrophils: Relevance in Inflammatory and Infectious Diseases.Cells. 2022 Apr 1;11(7):1188. doi: 10.3390/cells11071188. Cells. 2022. PMID: 35406754 Free PMC article. Review.
-
Loop Between NLRP3 Inflammasome and Reactive Oxygen Species.Antioxid Redox Signal. 2022 Apr;36(10-12):784-796. doi: 10.1089/ars.2020.8257. Epub 2022 Jan 17. Antioxid Redox Signal. 2022. PMID: 34538111 Review.
-
Does Pyroptosis Play a Role in Inflammasome-Related Disorders?Int J Mol Sci. 2022 Sep 9;23(18):10453. doi: 10.3390/ijms231810453. Int J Mol Sci. 2022. PMID: 36142364 Free PMC article. Review.
-
From Inflammasome to Exosome-Does Extracellular Vesicle Secretion Constitute an Inflammasome-Dependent Immune Response?Front Immunol. 2018 Sep 25;9:2188. doi: 10.3389/fimmu.2018.02188. eCollection 2018. Front Immunol. 2018. PMID: 30319640 Free PMC article. Review.
-
The zebrafish NLRP3 inflammasome has functional roles in ASC-dependent interleukin-1β maturation and gasdermin E-mediated pyroptosis.J Biol Chem. 2020 Jan 24;295(4):1120-1141. doi: 10.1074/jbc.RA119.011751. Epub 2019 Dec 18. J Biol Chem. 2020. PMID: 31852739 Free PMC article.
Cited by
-
NLRP3 Inflammasome in the Pathogenesis of Miscarriages.Int J Mol Sci. 2024 Sep 29;25(19):10513. doi: 10.3390/ijms251910513. Int J Mol Sci. 2024. PMID: 39408839 Free PMC article.
-
ATG16L1 in myeloid cells limits colorectal tumor growth in ApcMin/+ mice infected with colibactin-producing Escherichia coli via decreasing inflammasome activation.Autophagy. 2024 Oct;20(10):2186-2204. doi: 10.1080/15548627.2024.2359770. Epub 2024 Jun 10. Autophagy. 2024. PMID: 38818900 Free PMC article.
-
Immune cell contribution to vascular complications in diabetes.Front Endocrinol (Lausanne). 2025 May 21;16:1549945. doi: 10.3389/fendo.2025.1549945. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40469434 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

