Dental Follicle Stem Cells Promote Periodontal Regeneration through Periostin-Mediated Macrophage Infiltration and Reprogramming in an Inflammatory Microenvironment
- PMID: 37047322
- PMCID: PMC10094259
- DOI: 10.3390/ijms24076353
Dental Follicle Stem Cells Promote Periodontal Regeneration through Periostin-Mediated Macrophage Infiltration and Reprogramming in an Inflammatory Microenvironment
Abstract
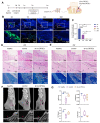
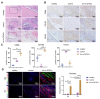
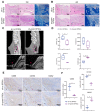
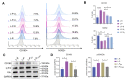
Dental follicle stem cells (DFSCs) have been verified to promote periodontal regeneration in an inflammatory microenvironment. When coping with inflammatory stimulation, DFSCs highly express periostin, a bioactive molecule closely related to periodontal homeostasis. It is worth exploring whether and how periostin plays a role in the promotion of periodontal regeneration by DFSCs. By tracking the fate of DFSCs, it was found that DFSCs significantly contributed to periodontal regeneration in rat periodontal defects while they had a low survival rate. They highly expressed periostin and improved the immune microenvironment in the defect area, especially via the recruitment and reprogramming of macrophages. Silencing periostin attenuated the effects of DFSCs in promoting periodontal regeneration and regulating macrophages. Recombinant human periostin (rhPeriostin) could not only directly promote macrophage reprogramming through the integrin αM/phosphorylated extracellular signal-regulated kinase (p-Erk)/Erk signaling pathway, but it also exhibited the potential to promote periodontal regeneration in rats when loaded in a collagen matrix. These results indicated that periostin is actively involved in the process by which DFSCs promote periodontal regeneration through the regulation of macrophages and is a promising molecular agent to promote periodontal regeneration. This study provides new insight into the mechanism by which DFSCs promote periodontal regeneration and suggests a new approach for periodontal regeneration therapy.
Keywords: dental follicle stem cells; macrophage reprogramming; periodontal regeneration; periostin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
LPS pretreated dental follicle stem cell derived exosomes promote periodontal tissue regeneration via miR-184 and PPARα-Akt-JNK signaling pathway.Stem Cell Res Ther. 2025 Jul 2;16(1):347. doi: 10.1186/s13287-025-04462-8. Stem Cell Res Ther. 2025. PMID: 40605003 Free PMC article.
-
Human dental follicle cell-derived conditioned media enhance periodontal regeneration by regulating the osteogenic differentiation and inflammation of periodontal ligament stem cells and macrophage polarization.Mol Cell Biochem. 2025 Jul;480(7):4431-4448. doi: 10.1007/s11010-025-05260-9. Epub 2025 Apr 2. Mol Cell Biochem. 2025. PMID: 40175780
-
Single-cell transcriptomics identifies PDGFRA+ progenitors orchestrating angiogenesis and periodontal tissue regeneration.Int J Oral Sci. 2025 Jul 24;17(1):56. doi: 10.1038/s41368-025-00384-6. Int J Oral Sci. 2025. PMID: 40707447 Free PMC article.
-
Enamel matrix derivative (Emdogain) for periodontal tissue regeneration in intrabony defects. A Cochrane systematic review.Eur J Oral Implantol. 2009 Winter;2(4):247-66. Eur J Oral Implantol. 2009. PMID: 20467602
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Mesenchymal Stem Cells in Soft Tissue Regenerative Medicine: A Comprehensive Review.Medicina (Kaunas). 2023 Aug 10;59(8):1449. doi: 10.3390/medicina59081449. Medicina (Kaunas). 2023. PMID: 37629738 Free PMC article. Review.
-
The landscape of cell regulatory and communication networks in the human dental follicle.Front Bioeng Biotechnol. 2025 Feb 5;13:1535245. doi: 10.3389/fbioe.2025.1535245. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 39974190 Free PMC article.
-
A ROS-responsive hydrogel incorporated with dental follicle stem cell-derived small extracellular vesicles promotes dental pulp repair by ameliorating oxidative stress.Bioact Mater. 2024 Jul 5;36:524-540. doi: 10.1016/j.bioactmat.2024.06.036. eCollection 2024 Jun. Bioact Mater. 2024. PMID: 39072284 Free PMC article.
-
Therapeutic effect of mesenchymal stem cell-derived exosome therapy for periodontal regeneration: a systematic review and meta-analysis of preclinical trials.J Orthop Surg Res. 2025 Jan 9;20(1):27. doi: 10.1186/s13018-024-05403-6. J Orthop Surg Res. 2025. PMID: 39780243 Free PMC article.
-
Advances in the research of immunomodulatory mechanism of mesenchymal stromal/stem cells on periodontal tissue regeneration.Front Immunol. 2025 Jan 3;15:1449411. doi: 10.3389/fimmu.2024.1449411. eCollection 2024. Front Immunol. 2025. PMID: 39830512 Free PMC article. Review.
References
-
- Yan N., Hu B., Xu J., Cai R., Liu Z., Fu D., Huo B., Liu Z., Zhao Y., Chen C., et al. Stem cell Janus patch for periodontal regeneration. Nano Today. 2022;42:101336. doi: 10.1016/j.nantod.2021.101336. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

