Involvement of Bcl-2 Family Proteins in Tetraploidization-Related Senescence
- PMID: 37047342
- PMCID: PMC10094710
- DOI: 10.3390/ijms24076374
Involvement of Bcl-2 Family Proteins in Tetraploidization-Related Senescence
Abstract
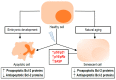
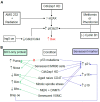
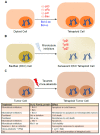
The B-cell lymphoma 2 (Bcl-2) family of proteins is the main regulator of apoptosis. However, multiple emerging evidence has revealed that Bcl-2 family proteins are also involved in cellular senescence. On the one hand, the different expression of these proteins determines the entry into senescence. On the other hand, entry into senescence modulates the expression of these proteins, generally conferring resistance to apoptosis. With some exceptions, senescent cells are characterized by the upregulation of antiapoptotic proteins and downregulation of proapoptotic proteins. Under physiological conditions, freshly formed tetraploid cells die by apoptosis due to the tetraploidy checkpoint. However, suppression of Bcl-2 associated x protein (Bax), as well as overexpression of Bcl-2, favors the appearance and survival of tetraploid cells. Furthermore, it is noteworthy that our laboratory has shown that the joint absence of Bax and Bcl-2 antagonist/killer (Bak) favors the entry into senescence of tetraploid cells. Certain microtubule inhibitory chemotherapies, such as taxanes and vinca alkaloids, induce the generation of tetraploid cells. Moreover, the combined use of inhibitors of antiapoptotic proteins of the Bcl-2 family with microtubule inhibitors increases their efficacy. In this review, we aim to shed light on the involvement of the Bcl-2 family of proteins in the senescence program activated after tetraploidization and the possibility of using this knowledge to create a new therapeutic strategy targeting cancer cells.
Keywords: Bcl-2 family proteins; apoptosis; cancer; senescence; tetraploidy.
Conflict of interest statement
G.K. holds research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Tollys and Vascage. G.K. is on the Board of Directors of the Bristol Myers Squibb Foundation France. G.K. is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. G.K. is on the scientific advisory boards of Hevolution, Institut Servier and Longevity Vision Funds. G.K. is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders. G.K.’s wife, Laurence Zitvogel, holds research contracts with Glaxo Smyth Kline, Incyte, Lytix, Kaleido, Innovate Pharma, Daiichi Sankyo, Pilege, Merus, Transgene, 9 m, Tusk and Roche, was on the on the Board of Directors of Transgene, is a cofounder of everImmune and holds patents covering the treatment of cancer and the therapeutic manipulation of microbiota. G.K.’s brother, Romano Kroemer, was an employee of Sanofi and now consults for Boehringer-Ingelheim. The funders had no role in the design of the study, in the writing of the manuscript or in the decision to publish the results. The rest of the authors declare no conflict of interest.
Figures

Similar articles
-
The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner.J Biol Chem. 2011 Mar 18;286(11):9382-92. doi: 10.1074/jbc.M110.203638. Epub 2010 Dec 9. J Biol Chem. 2011. PMID: 21148306 Free PMC article.
-
Paradoxical implication of BAX/BAK in the persistence of tetraploid cells.Cell Death Dis. 2021 Nov 1;12(11):1039. doi: 10.1038/s41419-021-04321-3. Cell Death Dis. 2021. PMID: 34725331 Free PMC article.
-
Prosurvival Bcl-2 family members affect autophagy only indirectly, by inhibiting Bax and Bak.Proc Natl Acad Sci U S A. 2014 Jun 10;111(23):8512-7. doi: 10.1073/pnas.1406425111. Epub 2014 May 27. Proc Natl Acad Sci U S A. 2014. PMID: 24912196 Free PMC article.
-
B-cell lymphoma-2 family proteins in the crosshairs: Small molecule inhibitors and activators for cancer therapy.Med Res Rev. 2024 Mar;44(2):707-737. doi: 10.1002/med.21999. Epub 2023 Nov 20. Med Res Rev. 2024. PMID: 37983840 Review.
-
BCL-2 proteins and apoptosis: Recent insights and unknowns.Biochem Biophys Res Commun. 2018 May 27;500(1):26-34. doi: 10.1016/j.bbrc.2017.06.190. Epub 2017 Jul 1. Biochem Biophys Res Commun. 2018. PMID: 28676391 Review.
Cited by
-
The Pivotal Role of One-Carbon Metabolism in Neoplastic Progression During the Aging Process.Biomolecules. 2024 Oct 31;14(11):1387. doi: 10.3390/biom14111387. Biomolecules. 2024. PMID: 39595564 Free PMC article. Review.
-
Tetraploidy in normal tissues and diseases: mechanisms and consequences.Chromosoma. 2025 Mar 21;134(1):3. doi: 10.1007/s00412-025-00829-1. Chromosoma. 2025. PMID: 40117022 Free PMC article. Review.
-
Blockade of interleukin-6 trans-signaling prevents mitochondrial dysfunction and cellular senescence in retinal endothelial cells.Exp Eye Res. 2023 Dec;237:109721. doi: 10.1016/j.exer.2023.109721. Epub 2023 Nov 11. Exp Eye Res. 2023. PMID: 37956941 Free PMC article.
-
Polyploidy and mTOR signaling: a possible molecular link.Cell Commun Signal. 2024 Mar 27;22(1):196. doi: 10.1186/s12964-024-01526-9. Cell Commun Signal. 2024. PMID: 38539200 Free PMC article. Review.
-
Anticancer potential of isoalantolactone in testicular cancer: an analysis of cytotoxicity, apoptosis, and signaling pathways.Aging (Albany NY). 2024 Oct 9;16(19):12820-12832. doi: 10.18632/aging.206076. Epub 2024 Oct 9. Aging (Albany NY). 2024. PMID: 39382942 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

