A Novel RT-LAMP for the Detection of Different Genotypes of Crimean-Congo Haemorrhagic Fever Virus in Patients from Spain
- PMID: 37047384
- PMCID: PMC10094476
- DOI: 10.3390/ijms24076411
A Novel RT-LAMP for the Detection of Different Genotypes of Crimean-Congo Haemorrhagic Fever Virus in Patients from Spain
Abstract
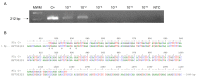
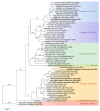
Crimean-Congo haemorrhagic fever (CCHF) is a potentially lethal tick-borne viral disease with a wide distribution. In Spain, 12 human cases of CCHF have been confirmed, with four deaths. The diagnosis of CCHF is hampered by the nonspecific symptoms, the high genetic diversity of CCHFV, and the biosafety requirements to manage the virus. RT-qPCR and serological tests are used for diagnosis with limitations. Reverse-transcription loop-mediated isothermal amplification (RT-LAMP) could be an effective alternative in the diagnosis of the disease. However, none of the few RT-LAMP assays developed to date has detected different CCHFV genotypes. Here, we designed a RT-LAMP using a degenerate primer set to compensate for the variability of the CCHFV target sequence. RT-LAMP was performed in colorimetric and real-time tests on RT-qPCR-confirmed CCHF patient samples notified in Spain in 2020 and 2021. Urine from an inpatient was analysed by RT-LAMP for the first time and compared with RT-qPCR. The amplicons obtained by RT-qPCR were sequenced and African III and European V genotypes were identified. RT-LAMP amplified both genotypes and was more sensitive than RT-qPCR in urine samples. We have developed a novel, rapid, specific, and sensitive RT-LAMP test that allows the detection of different CCHFV genotypes in clinical samples. This pan-CCHFV RT-LAMP detected viral RNA for the first time in urine samples. It can be easily performed as a single-tube isothermal colorimetric method on a portable platform in real time and without the need for expensive equipment, thus bringing molecular diagnostics closer to rural or resource-poor areas, where CCHF usually occurs.
Keywords: CCHFV; Crimean–Congo haemorrhagic fever virus; RT-LAMP; Spain; genotypes.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
MeSH terms
Supplementary concepts
Grants and funding
- PI22/01721/Instituto de Salud Carlos III
- J.G.-B.D./Predoctoral Fellowship Program of University of Salamanca and Santander Bank
- BDNS (Identif.): 422058, B.F.-S/Predoctoral Fellowship Program of Junta de Castilla y León cofunded by Fondo Social Europeo
- BDNS (Identif.): 487971, B.C.-V./Predoctoral Fellowship Program of Junta de Castilla y León cofunded by Fondo Social Europeo
LinkOut - more resources
Full Text Sources

