Uncovering the Underworld of Axial Spondyloarthritis
- PMID: 37047435
- PMCID: PMC10095023
- DOI: 10.3390/ijms24076463
Uncovering the Underworld of Axial Spondyloarthritis
Abstract
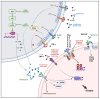
Axial spondyloarthritis (axial-SpA) is a multifactorial disease characterized by inflammation in sacroiliac joints and spine, bone reabsorption, and aberrant bone deposition, which may lead to ankylosis. Disease pathogenesis depends on genetic, immunological, mechanical, and bioenvironmental factors. HLA-B27 represents the most important genetic factor, although the disease may also develop in its absence. This MHC class I molecule has been deeply studied from a molecular point of view. Different theories, including the arthritogenic peptide, the unfolded protein response, and HLA-B27 homodimers formation, have been proposed to explain its role. From an immunological point of view, a complex interplay between the innate and adaptive immune system is involved in disease onset. Unlike other systemic autoimmune diseases, the innate immune system in axial-SpA has a crucial role marked by abnormal activity of innate immune cells, including γδ T cells, type 3 innate lymphoid cells, neutrophils, and mucosal-associated invariant T cells, at tissue-specific sites prone to the disease. On the other hand, a T cell adaptive response would seem involved in axial-SpA pathogenesis as emphasized by several studies focusing on TCR low clonal heterogeneity and clonal expansions as well as an interindividual sharing of CD4/8 T cell receptors. As a result of this immune dysregulation, several proinflammatory molecules are produced following the activation of tangled intracellular pathways involved in pathomechanisms of axial-SpA. This review aims to expand the current understanding of axial-SpA pathogenesis, pointing out novel molecular mechanisms leading to disease development and to further investigate potential therapeutic targets.
Keywords: axial spondyloarthritis; biological agents; cytokines; innate immunity; personalized medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sieper J., van der Heijde D., Landewé R., Brandt J., Burgos-Vagas R., Collantes-Estevez E., Dijkmans B., Dougados M., Khan M.A., Leirisalo-Repo M., et al. New Criteria for Inflammatory Back Pain in Patients with Chronic Back Pain: A Real Patient Exercise by Experts from the Assessment of SpondyloArthritis International Society (ASAS) Ann. Rheum. Dis. 2009;68:784–788. doi: 10.1136/ard.2008.101501. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

