Oncostatin M-Enriched Small Extracellular Vesicles Derived from Mesenchymal Stem Cells Prevent Isoproterenol-Induced Fibrosis and Enhance Angiogenesis
- PMID: 37047440
- PMCID: PMC10095085
- DOI: 10.3390/ijms24076467
Oncostatin M-Enriched Small Extracellular Vesicles Derived from Mesenchymal Stem Cells Prevent Isoproterenol-Induced Fibrosis and Enhance Angiogenesis
Abstract
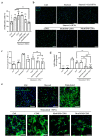
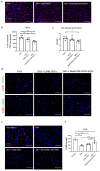
Myocardial fibrosis is a pathological hallmark of cardiac dysfunction. Oncostatin M (OSM) is a pleiotropic cytokine that can promote fibrosis in different organs after sustained exposure. However, OSM released by macrophages during cardiac fibrosis suppresses cardiac fibroblast activation by modulating transforming growth factor beta 1 (TGF-β1) expression and extracellular matrix deposition. Small extracellular vesicles (SEVs) from mesenchymal stromal cells (MSCs) are being investigated to treat myocardial infarction, using different strategies to bolster their therapeutic ability. Here, we generated TERT-immortalized human MSC cell lines (MSC-T) engineered to overexpress two forms of cleavage-resistant OSM fused to CD81TM (OSM-SEVs), which allows the display of the cytokine at the surface of secreted SEVs. The therapeutic potential of OSM-SEVs was assessed in vitro using human cardiac ventricular fibroblasts (HCF-Vs) activated by TGF-β1. Compared with control SEVs, OSM-loaded SEVs reduced proliferation in HCF-V and blunted telo-collagen expression. When injected intraperitoneally into mice treated with isoproterenol, OSM-loaded SEVs reduced fibrosis, prevented cardiac hypertrophy, and increased angiogenesis. Overall, we demonstrate that the enrichment of functional OSM on the surface of MSC-T-SEVs increases their potency in terms of anti-fibrotic and pro-angiogenic properties, which opens new perspectives for this novel biological product in cell-free-based therapies.
Keywords: Oncostatin M; extracellular vesicles; fibrosis; isoproterenol; mesenchymal stem cells.
Conflict of interest statement
Sandra Tejedor, Marc Buigues, Hernán Gonzále-King, Nahuel A. Garcia, and Pilar Sepúlveda declare competing financial interests, as the results of this manuscript are included in “Small extracellular vesicles with antifibrotic properties” registered by European Patent (application number EP22383291.6), 27 December 2022. Hernán González-King, Andreia M. Silva and Niek Dekker are or have been employees of Astrazeneca AB R&D and this work was conducted as part of their work activities. All authors have read the journal´s policy on conflict of interest.
Figures




References
-
- Arslan F., Lai R.C., Smeets M.B., Akeroyd L., Choo A., Aguor E.N.E., Timmers L., van Rijen H.V., Doevendans P.A., Pasterkamp G., et al. Mesenchymal Stem Cell-Derived Exosomes Increase ATP Levels, Decrease Oxidative Stress and Activate PI3K/Akt Pathway to Enhance Myocardial Viability and Prevent Adverse Remodeling after Myocardial Ischemia/Reperfusion Injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

