Triple Negative Breast Cancer Preclinical Therapeutic Management by a Cationic Ruthenium-Based Nucleolipid Nanosystem
- PMID: 37047448
- PMCID: PMC10094725
- DOI: 10.3390/ijms24076473
Triple Negative Breast Cancer Preclinical Therapeutic Management by a Cationic Ruthenium-Based Nucleolipid Nanosystem
Abstract
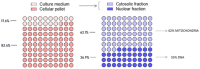
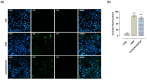
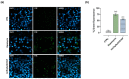
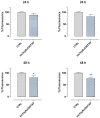
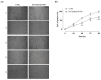
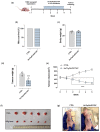
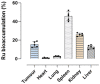
Based on compelling preclinical evidence concerning the progress of our novel ruthenium-based metallotherapeutics, we are focusing research efforts on challenging indications for the treatment of invasive neoplasms such as the triple-negative breast cancer (TNBC). This malignancy mainly afflicts younger women, who are black, or who have a BRCA1 mutation. Because of faster growing and spreading, TNBC differs from other invasive breast cancers having fewer treatment options and worse prognosis, where existing therapies are mostly ineffective, resulting in a large unmet biomedical need. In this context, we benefited from an experimental model of TNBC both in vitro and in vivo to explore the effects of a biocompatible cationic liposomal nanoformulation, named HoThyRu/DOTAP, able to effectively deliver the antiproliferative ruthenium(III) complex AziRu, thus resulting in a prospective candidate drug. As part of the multitargeting mechanisms featuring metal-based therapeutics other than platinum-containing agents, we herein validate the potential of HoThyRu/DOTAP liposomes to act as a multimodal anticancer agent through inhibition of TNBC cell growth and proliferation, as well as migration and invasion. The here-obtained preclinical findings suggest a potential targeting of the complex pathways network controlling invasive and migratory cancer phenotypes. Overall, in the field of alternative chemotherapy to platinum-based drugs, these outcomes suggest prospective brand-new settings for the nanostructured AziRu complex to get promising goals for the treatment of metastatic TNBC.
Keywords: DOTAP liposome; anticancer activity; cell migration and invasion; nucleolipid nanosystem; preclinical investigations; ruthenium(III) complex; triple-negative breast cancer (TNBC).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Breast Cancer Chemotherapeutic Options: A General Overview on the Preclinical Validation of a Multi-Target Ruthenium(III) Complex Lodged in Nucleolipid Nanosystems.Cells. 2020 Jun 5;9(6):1412. doi: 10.3390/cells9061412. Cells. 2020. PMID: 32517101 Free PMC article. Review.
-
Exploring cellular uptake, accumulation and mechanism of action of a cationic Ru-based nanosystem in human preclinical models of breast cancer.Sci Rep. 2019 May 7;9(1):7006. doi: 10.1038/s41598-019-43411-3. Sci Rep. 2019. PMID: 31065032 Free PMC article.
-
Cellular responses of BRCA1-defective and triple-negative breast cancer cells and in vitro BRCA1 interactions induced by metallo-intercalator ruthenium(II) complexes containing chloro-substituted phenylazopyridine.BMC Cancer. 2014 Feb 7;14:73. doi: 10.1186/1471-2407-14-73. BMC Cancer. 2014. PMID: 24507701 Free PMC article.
-
Safety and Efficacy Evaluation In Vivo of a Cationic Nucleolipid Nanosystem for the Nanodelivery of a Ruthenium(III) Complex with Superior Anticancer Bioactivity.Cancers (Basel). 2021 Oct 14;13(20):5164. doi: 10.3390/cancers13205164. Cancers (Basel). 2021. PMID: 34680314 Free PMC article.
-
BRCA1-Associated Triple-Negative Breast Cancer and Potential Treatment for Ruthenium-Based Compounds.Curr Cancer Drug Targets. 2016;16(7):606-17. doi: 10.2174/1568009616666160203113957. Curr Cancer Drug Targets. 2016. PMID: 26845433 Review.
Cited by
-
Design, Synthesis, and Characterization of an Amphiphilic Lipoic Acid-Based Ru(III) Complex as a Versatile Tool for the Functionalization of Different Nanosystems.Molecules. 2023 Jul 31;28(15):5775. doi: 10.3390/molecules28155775. Molecules. 2023. PMID: 37570744 Free PMC article.
-
Metal-Based Complexes in Cancer.Int J Mol Sci. 2023 Apr 14;24(8):7289. doi: 10.3390/ijms24087289. Int J Mol Sci. 2023. PMID: 37108457 Free PMC article.
-
Ferroptosis Among the Antiproliferative Pathways Activated by a Lipophilic Ruthenium(III) Complex as a Candidate Drug for Triple-Negative Breast Cancer.Pharmaceutics. 2025 Jul 16;17(7):918. doi: 10.3390/pharmaceutics17070918. Pharmaceutics. 2025. PMID: 40733126 Free PMC article.
-
Synthesis, Structure, and Stability of Copper(II) Complexes Containing Imidazoline-Phthalazine Ligands with Potential Anticancer Activity.Pharmaceuticals (Basel). 2025 Mar 6;18(3):375. doi: 10.3390/ph18030375. Pharmaceuticals (Basel). 2025. PMID: 40143151 Free PMC article.
-
Fatty Acid Synthase as Interacting Anticancer Target of the Terpenoid Myrianthic Acid Disclosed by MS-Based Proteomics Approaches.Int J Mol Sci. 2024 May 29;25(11):5918. doi: 10.3390/ijms25115918. Int J Mol Sci. 2024. PMID: 38892106 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

