Reactive Oxygen Species in the Aorta and Perivascular Adipose Tissue Precedes Endothelial Dysfunction in the Aorta of Mice with a High-Fat High-Sucrose Diet and Additional Factors
- PMID: 37047458
- PMCID: PMC10095299
- DOI: 10.3390/ijms24076486
Reactive Oxygen Species in the Aorta and Perivascular Adipose Tissue Precedes Endothelial Dysfunction in the Aorta of Mice with a High-Fat High-Sucrose Diet and Additional Factors
Abstract
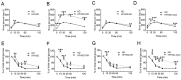
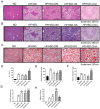
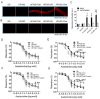
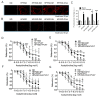
Metabolic syndrome (Mets) is the major contributor to the onset of metabolic complications, such as hypertension, type 2 diabetes mellitus (DM), dyslipidemia, and non-alcoholic fatty liver disease, resulting in cardiovascular diseases. C57BL/6 mice on a high-fat and high-sucrose diet (HFHSD) are a well-established model of Mets but have minor endothelial dysfunction in isolated aortas without perivascular adipose tissue (PVAT). The purpose of this study was to evaluate the effects of additional factors such as DM, dyslipidemia, and steatohepatitis on endothelial dysfunction in aortas without PVAT. Here, we employed eight-week-old male C57BL/6 mice fed with a normal diet (ND), HFHSD, steatohepatitis choline-deficient HFHSD (HFHSD-SH), and HFHSD containing 1% cholesterol and 0.1% deoxycholic acid (HFHSD-Chol) for 16 weeks. At week 20, some HFHSD-fed mice were treated with streptozocin to develop diabetes (HFHSD-DM). In PVAT-free aortas, the endothelial-dependent relaxation (EDR) did not differ between ND and HFHSD (p = 0.25), but in aortas with PVAT, the EDR of HFHSD-fed mice was impaired compared with ND-fed mice (p = 0.005). HFHSD-DM, HFHSD-SH, and HFHSD-Chol impaired the EDR in aortas without PVAT (p < 0.001, p = 0.019, and p = 0.009 vs. ND, respectively). Furthermore, tempol rescued the EDR in those models. In the Mets model, the EDR is compromised by PVAT, but with the addition of DM, dyslipidemia, and SH, the vessels themselves may result in impaired EDR.
Keywords: endothelial dysfunction; hypercholesterolemia; oxidative stress; perivascular adipose tissue; steatohepatitis; type 2 diabetes mellitus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Nox2 mediates high fat high sucrose diet-induced nitric oxide dysfunction and inflammation in aortic smooth muscle cells.J Mol Cell Cardiol. 2014 Jul;72:56-63. doi: 10.1016/j.yjmcc.2014.02.019. Epub 2014 Mar 11. J Mol Cell Cardiol. 2014. PMID: 24631774 Free PMC article.
-
High-fat/high-sucrose diet results in a high rate of MASH with HCC in a mouse model of human-like bile acid composition.Hepatol Commun. 2024 Dec 11;9(1):e0606. doi: 10.1097/HC9.0000000000000606. eCollection 2025 Jan 1. Hepatol Commun. 2024. PMID: 39670881 Free PMC article.
-
Timing of Medium-Chain Triglyceride Consumption Modulates Effects in Mice with Obesity Induced by a High-Fat High-Sucrose Diet.Nutrients. 2022 Dec 1;14(23):5096. doi: 10.3390/nu14235096. Nutrients. 2022. PMID: 36501131 Free PMC article.
-
The influence of perivascular adipose tissue on vascular homeostasis.Vasc Health Risk Manag. 2013;9:105-16. doi: 10.2147/VHRM.S33760. Epub 2013 Mar 28. Vasc Health Risk Manag. 2013. PMID: 23576873 Free PMC article. Review.
-
Perivascular Adipose Tissue Oxidative Stress on the Pathophysiology of Cardiometabolic Diseases.Curr Hypertens Rev. 2020;16(3):192-200. doi: 10.2174/1573402115666190410153634. Curr Hypertens Rev. 2020. PMID: 30968777 Review.
Cited by
-
Endothelial Reactive Oxygen Species: Key Players in Cardiovascular Health and Disease.Antioxid Redox Signal. 2025 Jun;42(16-18):905-932. doi: 10.1089/ars.2024.0706. Epub 2024 Sep 30. Antioxid Redox Signal. 2025. PMID: 39213161 Review.
-
Perivascular Adipose Tissue and Perivascular Adipose Tissue-Derived Extracellular Vesicles: New Insights in Vascular Disease.Cells. 2024 Aug 6;13(16):1309. doi: 10.3390/cells13161309. Cells. 2024. PMID: 39195199 Free PMC article. Review.
References
-
- Sato A., Yumita Y., Kagami K., Ishinoda Y., Kimura T., Osaki A., Toya T., Namba T., Endo S., Ido Y., et al. Endothelial Extracellular Signal-Regulated Kinase/Thromboxane A2/Prostanoid Receptor Pathway Aggravates Endothelial Dysfunction and Insulin Resistance in a Mouse Model of Metabolic Syndrome. J. Am. Heart. Assoc. 2022;11:e027538. doi: 10.1161/JAHA.122.027538. - DOI - PMC - PubMed
-
- Kujiraoka T., Satoh Y., Ayaori M., Shiraishi Y., Arai-Nakaya Y., Hakuno D., Yada H., Kuwada N., Endo S., Isoda K., et al. Hepatic extracellular signal-regulated kinase 2 suppresses endoplasmic reticulum stress and protects from oxidative stress and endothelial dysfunction. J. Am. Heart Assoc. 2013;2:e000361. doi: 10.1161/JAHA.113.000361. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

