Biofilm-Based Biocatalysis for Galactooligosaccharides Production by the Surface Display of β-Galactosidase in Pichia pastoris
- PMID: 37047479
- PMCID: PMC10094928
- DOI: 10.3390/ijms24076507
Biofilm-Based Biocatalysis for Galactooligosaccharides Production by the Surface Display of β-Galactosidase in Pichia pastoris
Abstract
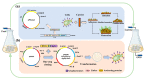
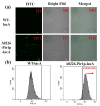
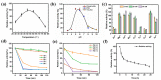
Galactooligosaccharides (GOS) are one of the most important functional oligosaccharide prebiotics. The surface display of enzymes was considered one of the most excellent strategies to obtain these products. However, a rough industrial environment would affect the biocatalytic process. The catalytic process could be efficiently improved using biofilm-based fermentation with high resistance and activity. Therefore, the combination of the surface display of β-galactosidase and biofilm formation in Pichia pastoris was constructed. The results showed that the catalytic conversion rate of GOS was up to 50.3% with the maximum enzyme activity of 5125 U/g by screening the anchorin, and the number of the continuous catalysis batches was up to 23 times. Thus, surface display based on biofilm-immobilized fermentation integrated catalysis and growth was a co-culture system, such that a dynamic equilibrium in the consolidated integrative process was achieved. This study provides the basis for developing biofilm-based surface display methods in P. pastoris during biochemical production processes.
Keywords: Pichia pastoris; biofilm; galactooligosaccharides; yeast surface display; β-galactosidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Improving the Secretion Yield of the β-Galactosidase Bgal1-3 in Pichia pastoris for Use as a Potential Catalyst in the Production of Prebiotic-Enriched Milk.J Agric Food Chem. 2017 Dec 13;65(49):10757-10766. doi: 10.1021/acs.jafc.7b04694. Epub 2017 Dec 5. J Agric Food Chem. 2017. PMID: 29181978
-
Characterization of Sulfolobus solfataricus β-galactosidase mutant F441Y expressed in Pichia pastoris.J Sci Food Agric. 2014 May;94(7):1359-65. doi: 10.1002/jsfa.6419. Epub 2013 Nov 1. J Sci Food Agric. 2014. PMID: 24114556
-
A method for the production of D-tagatose using a recombinant Pichia pastoris strain secreting β-D-galactosidase from Arthrobacter chlorophenolicus and a recombinant L-arabinose isomerase from Arthrobacter sp. 22c.Microb Cell Fact. 2012 Aug 23;11:113. doi: 10.1186/1475-2859-11-113. Microb Cell Fact. 2012. PMID: 22917022 Free PMC article.
-
Secretory expression of human protein in the Yeast Pichia pastoris by controlled fermentor culture.Recent Pat Biotechnol. 2010 Jun;4(2):153-66. doi: 10.2174/187220810791110679. Recent Pat Biotechnol. 2010. PMID: 20180764 Review.
-
Towards molecular farming in the future: pichia pastoris-based production of single-chain antibody fragments.Biotechnol Appl Biochem. 1999 Oct;30(2):117-20. Biotechnol Appl Biochem. 1999. PMID: 10512790 Review.
Cited by
-
Biofilm-based biocatalysis for β-cyclodextrin production by the surface-display of β-cyclodextrin glycosyltransferase in Bacillus subtilis.Sci Rep. 2024 Dec 2;14(1):29925. doi: 10.1038/s41598-024-81490-z. Sci Rep. 2024. PMID: 39622869 Free PMC article.
-
Surface Display Technologies for Whole-Cell Biocatalysts: Advances in Optimization Strategies, Food Applications, and Future Perspectives.Foods. 2025 May 19;14(10):1803. doi: 10.3390/foods14101803. Foods. 2025. PMID: 40428582 Free PMC article. Review.
-
Freeze-cast SiOC ceramics supporting the growth of industrially relevant microorganisms.PLoS One. 2025 Jun 12;20(6):e0325311. doi: 10.1371/journal.pone.0325311. eCollection 2025. PLoS One. 2025. PMID: 40504811 Free PMC article.
References
-
- Tzortzis G., Vulevic J. Galacto-Oligosaccharide Prebiotics. In: Charalampopoulos D., Rastall R.A., editors. Prebiotics and Probiotics Science and Technology. Springer; New York, NY, USA: 2009. pp. 207–244.
-
- Schwab C., Lee V., Sørensen K.I., Gänzle M.G. Production of galactooligosaccharides and heterooligosaccharides with disrupted cell extracts and whole cells of lactic acid bacteria and bifidobacteria. Int. Dairy J. 2011;21:748–754. doi: 10.1016/j.idairyj.2011.04.010. - DOI
-
- Güleç H.A., Gürdaş S., Albayrak N., Mutlu M. Immobilization of Aspergillus oryzae β-galactosidase on low-pressure plasma-modified cellulose acetate membrane using polyethyleneimine for production of galactooligosaccharide. Biotechnol. Bioprocess Eng. 2010;15:1006–1015. doi: 10.1007/s12257-010-0046-7. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 2022YFC2105400/the National Key R&D Program of China
- 2021YFC2101100/the National Key R&D Program of China
- 22178176/the National Natural Science Foundation of China
- 2018YFB1501705/the National Key Research and Development Program of China
- 21636003/the key program of the National Natural Science Foundation of China
- IRT_14R28/the Program for Changjiang Scholars and Innovative Research Team in University
- ZDYF20200220/Key Research and Development Program of Nanjing Jiangbei New Area
- BE2019001/Key R & D plan of Jiangsu Province
- BK20220334/Youth Fund of Natural Science Foundation of Jiangsu Province
- 22208157/the National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources

