Cathepsin-L Secreted by High-Quality Bovine Embryos Exerts an Embryotrophic Effect In Vitro
- PMID: 37047535
- PMCID: PMC10094850
- DOI: 10.3390/ijms24076563
Cathepsin-L Secreted by High-Quality Bovine Embryos Exerts an Embryotrophic Effect In Vitro
Abstract
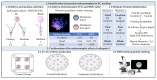
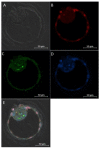
While human in vitro embryo production is generally performed individually, animal models have shown that culturing embryos in groups improves blastocyst yield and quality. Paracrine embryotrophins could be responsible for this improved embryo development, but their identity remains largely unknown. We hypothesize that supplementation of embryotrophic proteins to a culture medium could be the key to improve individual embryo production. In this study, proteomics screening of culture media conditioned by bovine embryos revealed cathepsin-L as being secreted by both excellent- and good-quality embryos, while being absent in the medium conditioned by poor-quality embryos. The embryotrophic role of cathepsin-L was explored in vitro, whereby bovine zygotes were cultured individually for 8 days with or without cathepsin-L. Preliminary dose-response experiments pointed out 100 ng/mL as the optimal concentration of cathepsin-L in embryo culture medium. Supplementation of cathepsin-L to individual culture systems significantly improved blastocyst development and quality in terms of blastocoel formation at day 7, and the hatching ratio and apoptotic cell ratio at day 8, compared to the control. Taken together, cathepsin-L acts as an important embryotrophin by increasing embryo quality, and regulating blastulation and hatching in bovine in vitro embryo production.
Keywords: embryonic development; embryotrophins; in vitro culture.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Evidence for the requirement of autocrine growth factors for development of mouse preimplantation embryos in vitro.Biol Reprod. 1997 Jan;56(1):229-37. doi: 10.1095/biolreprod56.1.229. Biol Reprod. 1997. PMID: 9002654
-
The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos.Reproduction. 2006 Feb;131(2):269-77. doi: 10.1530/rep.1.00677. Reproduction. 2006. PMID: 16452720
-
Different preferences of IVF and SCNT bovine embryos for culture media.Zygote. 2014 Feb;22(1):1-9. doi: 10.1017/S0967199412000184. Epub 2012 Jul 11. Zygote. 2014. PMID: 22784408
-
Effect of supplementation of different growth factors in embryo culture medium with a small number of bovine embryos on in vitro embryo development and quality.Animal. 2013 Mar;7(3):455-62. doi: 10.1017/S1751731112001991. Epub 2012 Nov 5. Animal. 2013. PMID: 23121725 Clinical Trial.
-
Supplementation of bovine embryo culture medium with L-arginine improves embryo quality via nitric oxide production.Mol Reprod Dev. 2014 Oct;81(10):918-27. doi: 10.1002/mrd.22387. Epub 2014 Sep 18. Mol Reprod Dev. 2014. PMID: 25236163
Cited by
-
In Vitro Culture of Mammalian Embryos: Is There Room for Improvement?Cells. 2024 Jun 7;13(12):996. doi: 10.3390/cells13120996. Cells. 2024. PMID: 38920627 Free PMC article. Review.
-
Pre-Implantation Bovine Embryo Evaluation-From Optics to Omics and Beyond.Animals (Basel). 2023 Jun 24;13(13):2102. doi: 10.3390/ani13132102. Animals (Basel). 2023. PMID: 37443900 Free PMC article. Review.
-
Cathepsin L regulates oocyte meiosis and preimplantation embryo development.Cell Prolif. 2024 Jan;57(1):e13526. doi: 10.1111/cpr.13526. Epub 2023 Jul 7. Cell Prolif. 2024. PMID: 37417221 Free PMC article.
-
Changes in the Transcription of Proliferation- and Apoptosis-Related Genes in Embryos in Women of Different Ages under the Influence of Extracellular Vesicles from Donor Follicular Fluid In Vitro.Bull Exp Biol Med. 2024 Mar;176(5):658-665. doi: 10.1007/s10517-024-06087-y. Epub 2024 May 10. Bull Exp Biol Med. 2024. PMID: 38727955
-
Pressing needs and recent advances to enhance production of embryos in vitro in cattle.Anim Reprod. 2024 Aug 26;21(3):e20240036. doi: 10.1590/1984-3143-AR2024-0036. eCollection 2024. Anim Reprod. 2024. PMID: 39286365 Free PMC article.
References
-
- Galli C., Duchi R., Colleoni S., Lagutina I., Lazzari G. Ovum Pick up, Intracytoplasmic Sperm Injection and Somatic Cell Nuclear Transfer in Cattle, Buffalo and Horses: From the Research Laboratory to Clinical Practice. Theriogenology. 2014;81:138–151. doi: 10.1016/j.theriogenology.2013.09.008. - DOI - PubMed
-
- Gerits I., Angel-Velez D., Ververs C., Govaere J., van Soom A., Smits K. First Blastocyst Production after ICSI with Zebra Semen. J. Equine Veter.-Sci. 2020;89:103053. doi: 10.1016/j.jevs.2020.103053. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

