A Comparative Study of Binding Interactions between Proteins and Flavonoids in Angelica Keiskei: Stability, α-Glucosidase Inhibition and Interaction Mechanisms
- PMID: 37047555
- PMCID: PMC10095106
- DOI: 10.3390/ijms24076582
A Comparative Study of Binding Interactions between Proteins and Flavonoids in Angelica Keiskei: Stability, α-Glucosidase Inhibition and Interaction Mechanisms
Abstract
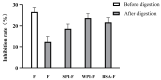
Flavonoids are easily destroyed and their activity lost during gastrointestinal digestion. Protein-based nanocomplexes, a delivery system that promotes nutrient stability and bioactivity, have received increasing attention in recent years. This study investigated the stability, inhibitory activity against α-glucosidase and interaction mechanisms of protein-based nanocomplexes combining whey protein isolate (WPI), soybean protein isolate (SPI) and bovine serum albumin (BSA) with flavonoids (F) from A. keiskei using spectrophotometry, fluorescence spectra and molecular docking approaches. The results show that the flavonoid content of WPI-F (23.17 ± 0.86 mg/g) was higher than those of SPI-F (19.41 ± 0.56 mg/g) and BSA-F (20.15 ± 0.62 mg/g) after simulated digestion in vitro. Furthermore, the inhibition rate of WPI-F (23.63 ± 0.02%) against α-glucosidase was also better than those of SPI-F (18.56 ± 0.02%) and BSA-F (21.62 ± 0.02%). The inhibition rate of WPI-F increased to nearly double that of F alone (12.43 ± 0.02%) (p < 0.05). Molecular docking results indicated that the protein-flavonoids (P-F) binding occurs primarily through hydrophobic forces, hydrogen bonds and ionic bonds. Thermodynamic analysis (ΔH > 0, ΔS > 0) indicated that the P-F interactions are predominantly hydrophobic forces. In addition, the absolute value of ΔG for WPI-F is greater (-30.22 ± 2.69 kJ mol-1), indicating that WPI-F releases more heat energy when synthesized and is more conducive to combination. This paper serves as a valuable reference for the stability and bioactivity of flavonoids from A. keiskei.
Keywords: A. keiskei; flavonoids; interaction mechanism; protein-based nanocomplex; stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Exploring the effects of whey protein components on the interaction and stability of cyanidin-3-O-glucoside.J Sci Food Agric. 2025 Jan 15;105(1):294-304. doi: 10.1002/jsfa.13828. Epub 2024 Aug 23. J Sci Food Agric. 2025. PMID: 39179519
-
Interaction mechanism of flavonoids with whey protein isolate: A spectrofluorometric and theoretical investigation.Food Chem. 2021 Sep 1;355:129617. doi: 10.1016/j.foodchem.2021.129617. Epub 2021 Mar 19. Food Chem. 2021. PMID: 33784543
-
Inhibitory mechanism of sinensetin on α-glucosidase and non-enzymatic glycation: Insights from spectroscopy and molecular docking analyses.Int J Biol Macromol. 2021 Jan 1;166:259-267. doi: 10.1016/j.ijbiomac.2020.10.174. Epub 2020 Oct 26. Int J Biol Macromol. 2021. PMID: 33115652
-
Interaction of Flavonoids from Woodwardia unigemmata with Bovine Serum Albumin (BSA): Application of Spectroscopic Techniques and Molecular Modeling Methods.Molecules. 2017 Aug 9;22(8):1317. doi: 10.3390/molecules22081317. Molecules. 2017. PMID: 28792461 Free PMC article.
-
[Binding interaction of harpagoside and bovine serum albumin: spectroscopic methodologies and molecular docking].Zhongguo Zhong Yao Za Zhi. 2018 Mar;43(5):993-1000. doi: 10.19540/j.cnki.cjcmm.2018.0031. Zhongguo Zhong Yao Za Zhi. 2018. PMID: 29676099 Chinese.
Cited by
-
Anti-Inflammatory Effects of a Novel Acetonitrile-Water Extract of Lens Culinaris against LPS-Induced Damage in Caco-2 Cells.Int J Mol Sci. 2024 Mar 28;25(7):3802. doi: 10.3390/ijms25073802. Int J Mol Sci. 2024. PMID: 38612611 Free PMC article.
-
The Role of Selected Flavonoids in Modulating Neuroinflammation in Alzheimer's Disease: Mechanisms and Therapeutic Potential.Brain Sci. 2025 May 5;15(5):485. doi: 10.3390/brainsci15050485. Brain Sci. 2025. PMID: 40426656 Free PMC article. Review.
References
-
- Kim D.W., Curtis-Long M.J., Yuk H.J., Wang Y., Song Y.H., Jeong S.H., Park K.H. Quantitative analysis of phenolic metabolites from different parts of Angelica keiskei by HPLC-ESI MS/MS and their xanthine oxidase inhibition. Food Chem. 2014;153:20–27. doi: 10.1016/j.foodchem.2013.12.026. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- CY202234/Zhejiang Provincial Administration for Market Regulation
- Y201942374/Scientific Research Fund of Education Department of Zhejiang Province
- 91856126/National Natural Science Foundation of China
- HZ202202/One health Interdisciplinary Research Project, Ningbo University
- NMKJXM202209-2/Science and Technology to Develop Mongolia
LinkOut - more resources
Full Text Sources

