Crosslinked Chitosan Nanoparticles with Muco-Adhesive Potential for Intranasal Delivery Applications
- PMID: 37047562
- PMCID: PMC10094788
- DOI: 10.3390/ijms24076590
Crosslinked Chitosan Nanoparticles with Muco-Adhesive Potential for Intranasal Delivery Applications
Abstract
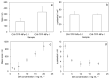
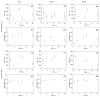
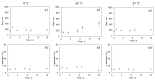
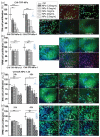
Intranasal drug delivery is convenient and provides a high bioavailability but requires the use of mucoadhesive nanocarriers. Chitosan is a well-established polymer for mucoadhesive applications but can suffer from poor cytocompatibility and stability upon administration. In this work, we present a method to obtain stable and cytocompatible crosslinked chitosan nanoparticles. We used 2,6-pyridinedicarboxylic acid as a biocompatible crosslinker and compared the obtained particles with those prepared by ionotropic gelation using sodium tripolyphosphate. Nanoparticles were tested to evaluate the size and the surface charge, as well as their stability in storage conditions (4 °C), at the nasal cavity temperature (32 °C), and at the body temperature (37 °C). The crosslinked chitosan nanoparticles showed a size around 150 nm and a surface charge of 10.3 mV ± 0.9 mV, both compatible with the intranasal drug administration. Size and surface charge parameters did not significantly vary over time, indicating the good stability of these nanoparticles. We finally tested their cytocompatibility in vitro using SHSY5Y human neuroblastoma and RPMI 2650 human nasal epithelial cells, with positive results. In conclusion, the proposed synthetic system shows an interesting potential as a drug carrier for intranasal delivery.
Keywords: chitosan nanoparticles; drug delivery; intranasal administration; mucoadhesion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Di Filippo L.D., Duarte J., Fonseca-Santos B., Tavares Júnior A.G., Araújo V., Roque Borda C.A., Chorilli M. Mucoadhesive nanosystems for nose-to-brain drug delivery in the treatment of central nervous system diseases. Curr. Med. Chem. 2022;29:3079–3110. doi: 10.2174/0929867328666210813154019. - DOI - PubMed
-
- Bayer I.S. Recent advances in mucoadhesive interface materials, mucoadhesion characterization, and technologies. Adv. Mater. Interf. 2022;9:2200211. doi: 10.1002/admi.202200211. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

