Delineation of G Protein-Coupled Receptor Kinase Phosphorylation Sites within the D1 Dopamine Receptor and Their Roles in Modulating β-Arrestin Binding and Activation
- PMID: 37047571
- PMCID: PMC10095280
- DOI: 10.3390/ijms24076599
Delineation of G Protein-Coupled Receptor Kinase Phosphorylation Sites within the D1 Dopamine Receptor and Their Roles in Modulating β-Arrestin Binding and Activation
Abstract
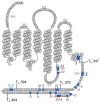
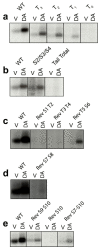
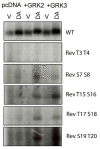
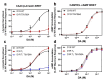
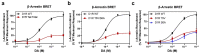
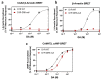
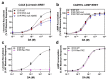
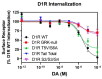
The D1 dopamine receptor (D1R) is a G protein-coupled receptor that signals through activating adenylyl cyclase and raising intracellular cAMP levels. When activated, the D1R also recruits the scaffolding protein β-arrestin, which promotes receptor desensitization and internalization, as well as additional downstream signaling pathways. These processes are triggered through receptor phosphorylation by G protein-coupled receptor kinases (GRKs), although the precise phosphorylation sites and their role in recruiting β-arrestin to the D1R remains incompletely described. In this study, we have used detailed mutational and in situ phosphorylation analyses to completely identify the GRK-mediated phosphorylation sites on the D1R. Our results indicate that GRKs can phosphorylate 14 serine and threonine residues within the C-terminus and the third intracellular loop (ICL3) of the receptor, and that this occurs in a hierarchical fashion, where phosphorylation of the C-terminus precedes that of the ICL3. Using β-arrestin recruitment assays, we identified a cluster of phosphorylation sites in the proximal region of the C-terminus that drive β-arrestin binding to the D1R. We further provide evidence that phosphorylation sites in the ICL3 are responsible for β-arrestin activation, leading to receptor internalization. Our results suggest that distinct D1R GRK phosphorylation sites are involved in β-arrestin binding and activation.
Keywords: D1 receptor; dopamine; phosphorylation; β-arrestin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

