Pegylated Gold Nanoparticles Conjugated with siRNA: Complexes Formation and Cytotoxicity
- PMID: 37047610
- PMCID: PMC10094790
- DOI: 10.3390/ijms24076638
Pegylated Gold Nanoparticles Conjugated with siRNA: Complexes Formation and Cytotoxicity
Abstract
Drug delivery systems such as dendrimers, liposomes, polymers or gold/silver nanoparticles could be used to advance modern medicine. One significant pharmacological problem is crossing biological barriers by commonly used drugs, e.g., in the treatment of neurodegenerative diseases, which have a problem of the blood-brain barrier (BBB) restricting drug delivery. Numerous studies have been conducted to find appropriate drug carriers that are safe, biocompatible and efficient. In this work, we evaluate pegylated gold nanoparticles AuNP14a and AuNP14b after their conjugation with therapeutic siRNA directed against APOE4. This genetic risk factor remains the strongest predictor for late-onset Alzheimer's disease. The study aimed to assess the biophysical properties of AuNPs/siAPOE complexes and to check their biological safety on healthy cells using human brain endothelial cells (HBEC-5i). Techniques such as fluorescence polarization, circular dichroism, dynamic light scattering, ζ-potential measurements and gel retardation assay showed that AuNPs form stable complexes with siRNA. Subsequently, cytotoxicity assays proved the biological safety of formed conjugates. Obtained results enabled us to find effective concentrations of AuNPs when complexes are formed and non-toxic for healthy cells. One of the studied nanoparticles, AuNP14b complexed with siRNA, displayed lower cytotoxicity (MTT assay, cells viability -74.8 ± 3.1%) than free nanoparticles (44.7 ± 3.6%). This may be promising for further investigations in nucleic acid delivery and could have practical use in treating neurodegenerative diseases.
Keywords: biophysical interaction; complex formation; cytotoxicity; gold nanoparticles; siRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
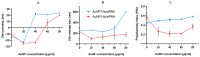
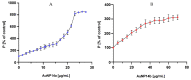

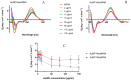

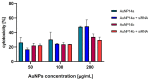

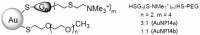
Similar articles
-
Pegylated gold nanoparticles interact with lipid bilayer and human serum albumin and transferrin.Sci Rep. 2024 Oct 18;14(1):24408. doi: 10.1038/s41598-024-74898-0. Sci Rep. 2024. PMID: 39420206 Free PMC article.
-
Evaluation of the physicochemical properties and the biocompatibility of polyethylene glycol-conjugated gold nanoparticles: A formulation strategy for siRNA delivery.Colloids Surf B Biointerfaces. 2015 Nov 1;135:604-612. doi: 10.1016/j.colsurfb.2015.08.032. Epub 2015 Aug 24. Colloids Surf B Biointerfaces. 2015. PMID: 26322474
-
PEGylation of Dendronized Gold Nanoparticles Affects Their Interaction with Thrombin and siRNA.J Phys Chem B. 2021 Feb 4;125(4):1196-1206. doi: 10.1021/acs.jpcb.0c10177. Epub 2021 Jan 22. J Phys Chem B. 2021. PMID: 33481607
-
Applications of Gold Nanoparticles in Brain Diseases across the Blood-Brain Barrier.Curr Med Chem. 2022;29(39):6063-6083. doi: 10.2174/0929867329666220527121943. Curr Med Chem. 2022. PMID: 35638273 Review.
-
Polymer decorated gold nanoparticles in nanomedicine conjugates.Adv Colloid Interface Sci. 2017 Nov;249:386-399. doi: 10.1016/j.cis.2017.01.007. Epub 2017 Feb 15. Adv Colloid Interface Sci. 2017. PMID: 28259207 Review.
Cited by
-
Assessing the toxicity of one-step-synthesized PEG-coated gold nanoparticles: in vitro and in vivo studies.Einstein (Sao Paulo). 2024 May 20;22:eAO0764. doi: 10.31744/einstein_journal/2024AO0764. eCollection 2024. Einstein (Sao Paulo). 2024. PMID: 38775605 Free PMC article.
-
Impacts of polyethylene glycol (PEG) dispersity on protein adsorption, pharmacokinetics, and biodistribution of PEGylated gold nanoparticles.RSC Adv. 2024 Jul 1;14(29):20757-20764. doi: 10.1039/d4ra03153a. eCollection 2024 Jun 27. RSC Adv. 2024. PMID: 38952930 Free PMC article.
-
Special Issue "Latest Advances in Nanomedicine Strategies for Different Diseases".Int J Mol Sci. 2024 May 27;25(11):5835. doi: 10.3390/ijms25115835. Int J Mol Sci. 2024. PMID: 38892023 Free PMC article.
-
Functional gold nanoparticles for analysis and delivery of nucleic acids.J Food Drug Anal. 2024 Sep 13;32(3):252-273. doi: 10.38212/2224-6614.3514. J Food Drug Anal. 2024. PMID: 39636773 Free PMC article. Review.
References
-
- Razavi R., Amiri M., Alshamsi H., Eslaminejad T., Salavati-Niasari M. Green synthesis of Ag nanoparticles in oil-in-water nano-emulsion and evaluation of their antibacterial and cytotoxic properties as well as molecular docking. Arab. J. Chem. 2021;14:103323. doi: 10.1016/j.arabjc.2021.103323. - DOI
-
- Biswas P., Polash S.A., Dey D., Kaium M.A., Mahmud A.R., Yasmin F., Baral S.K., Islam M.A., Rahaman T.I., Abdullah A., et al. Advanced implications of nanotechnology in disease control and environmental perspectives. Biomed. Pharmacother. 2023;158:114172. doi: 10.1016/j.biopha.2022.114172. - DOI - PubMed
-
- Patra J.K., Das G., Fraceto L.F., Ramos Campos E.V., del Pilar Rodriguez-Torres M., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. - DOI - PMC - PubMed
-
- Lu H., Wang J., Wang T., Zhong J., Bao Y., Hao H. Recent Progress on Nanostructures for Drug Delivery Applications. J. Nanomater. 2016;2016:5762431. doi: 10.1155/2016/5762431. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

