The Labyrinthine Landscape of APP Processing: State of the Art and Possible Novel Soluble APP-Related Molecular Players in Traumatic Brain Injury and Neurodegeneration
- PMID: 37047617
- PMCID: PMC10095589
- DOI: 10.3390/ijms24076639
The Labyrinthine Landscape of APP Processing: State of the Art and Possible Novel Soluble APP-Related Molecular Players in Traumatic Brain Injury and Neurodegeneration
Abstract
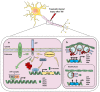
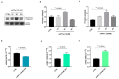
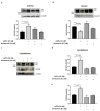
Amyloid Precursor Protein (APP) and its cleavage processes have been widely investigated in the past, in particular in the context of Alzheimer's Disease (AD). Evidence of an increased expression of APP and its amyloidogenic-related cleavage enzymes, β-secretase 1 (BACE1) and γ-secretase, at the hit axon terminals following Traumatic Brain Injury (TBI), firstly suggested a correlation between TBI and AD. Indeed, mild and severe TBI have been recognised as influential risk factors for different neurodegenerative diseases, including AD. In the present work, we describe the state of the art of APP proteolytic processing, underlining the different roles of its cleavage fragments in both physiological and pathological contexts. Considering the neuroprotective role of the soluble APP alpha (sAPPα) fragment, we hypothesised that sAPPα could modulate the expression of genes of interest for AD and TBI. Hence, we present preliminary experiments addressing sAPPα-mediated regulation of BACE1, Isthmin 2 (ISM2), Tetraspanin-3 (TSPAN3) and the Vascular Endothelial Growth Factor (VEGFA), each discussed from a biological and pharmacological point of view in AD and TBI. We finally propose a neuroprotective interaction network, in which the Receptor for Activated C Kinase 1 (RACK1) and the signalling cascade of PKCβII/nELAV/VEGF play hub roles, suggesting that vasculogenic-targeting therapies could be a feasible approach for vascular-related brain injuries typical of AD and TBI.
Keywords: BACE1; ELAV; ISM2; PKC; RACK1; TSPAN3; VEGF; secretase.
Conflict of interest statement
The authors declare no conflict of interests.
Figures




Similar articles
-
Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1(BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis.Eur J Neurosci. 2013 Jun;37(12):1962-9. doi: 10.1111/ejn.12235. Eur J Neurosci. 2013. PMID: 23773065
-
Alternative Selection of β-Site APP-Cleaving Enzyme 1 (BACE1) Cleavage Sites in Amyloid β-Protein Precursor (APP) Harboring Protective and Pathogenic Mutations within the Aβ Sequence.J Biol Chem. 2016 Nov 11;291(46):24041-24053. doi: 10.1074/jbc.M116.744722. Epub 2016 Sep 29. J Biol Chem. 2016. PMID: 27687728 Free PMC article.
-
Berberine Alleviates Amyloid-Beta Pathology in the Brain of APP/PS1 Transgenic Mice via Inhibiting β/γ-Secretases Activity and Enhancing α-Secretases.Curr Alzheimer Res. 2018;15(11):1045-1052. doi: 10.2174/1567205015666180702105740. Curr Alzheimer Res. 2018. PMID: 29962345
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Secretases in Alzheimer's disease: Novel insights into proteolysis of APP and TREM2.Curr Opin Neurobiol. 2022 Feb;72:101-110. doi: 10.1016/j.conb.2021.09.003. Epub 2021 Oct 22. Curr Opin Neurobiol. 2022. PMID: 34689040 Review.
Cited by
-
Endocrine Disrupting Toxicity of Bisphenol A and Its Analogs: Implications in the Neuro-Immune Milieu.J Xenobiot. 2025 Jan 17;15(1):13. doi: 10.3390/jox15010013. J Xenobiot. 2025. PMID: 39846545 Free PMC article. Review.
-
Disruption of Epithelial Barrier Integrity via Altered GILZ/c-Rel/RACK1 Signaling in Inflammatory Bowel Disease.J Crohns Colitis. 2025 Jan 11;19(1):jjae191. doi: 10.1093/ecco-jcc/jjae191. J Crohns Colitis. 2025. PMID: 39693354 Free PMC article.
-
Exploring the Impact of Mitoquinone Supplementation on Glycan Profiles in a Repeated Mild Traumatic Brain Injury Mouse Model.Neurotrauma Rep. 2025 Jun 16;6(1):525-538. doi: 10.1089/neur.2025.0054. eCollection 2025. Neurotrauma Rep. 2025. PMID: 40636441 Free PMC article.
-
Plasmalogens Improve Lymphatic Clearance of Amyloid Beta from Mouse Brain and Cognitive Functions.Int J Mol Sci. 2024 Nov 22;25(23):12552. doi: 10.3390/ijms252312552. Int J Mol Sci. 2024. PMID: 39684263 Free PMC article.
-
Upregulation of VEGFA through the adenosine A2A receptor is a crucial pathway for inhibiting pericyte apoptosis in chronic cerebral hypoperfusion.Sci Rep. 2025 Jul 4;15(1):23955. doi: 10.1038/s41598-025-08407-2. Sci Rep. 2025. PMID: 40615465 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

