1,25(OH)2D3 Differently Modulates the Secretory Activity of IFN-DC and IL4-DC: A Study in Cells from Healthy Donors and MS Patients
- PMID: 37047690
- PMCID: PMC10094841
- DOI: 10.3390/ijms24076717
1,25(OH)2D3 Differently Modulates the Secretory Activity of IFN-DC and IL4-DC: A Study in Cells from Healthy Donors and MS Patients
Abstract
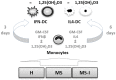
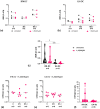
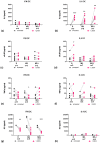
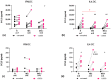
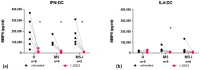
Immune mechanisms play an essential role in driving multiple sclerosis (MS) and altered trafficking and/or activation of dendritic cells (DC) were observed in the central nervous system and cerebrospinal fluid of MS patients. Interferon β (IFNβ) has been used as a first-line therapy in MS for almost three decades and vitamin D deficiency is a recognized environmental risk factor for MS. Both IFNβ and vitamin D modulate DC functions. Here, we studied the response to 1,25-dihydoxyvitamin D3 (1,25(OH)2D3) of DC obtained with IFNβ/GM-CSF (IFN-DC) compared to classically derived IL4-DC, in three donor groups: MS patients free of therapy, MS patients undergoing IFNβ therapy, and healthy donors. Except for a decreased CCL2 secretion by IL4-DC from the MS group, no major defects were observed in the 1,25(OH)2D3 response of either IFN-DC or IL4-DC from MS donors compared to healthy donors. However, the two cell models strongly differed for vitamin D receptor level of expression as well as for basal and 1,25(OH)2D3-induced cytokine/chemokine secretion. 1,25(OH)2D3 up-modulated IL6, its soluble receptor sIL6R, and CCL5 in IL4-DC, and down-modulated IL10 in IFN-DC. IFN-DC, but not IL4-DC, constitutively secreted high levels of IL8 and of matrix-metalloproteinase-9, both down-modulated by 1,25(OH)2D3. DC may contribute to MS pathogenesis, but also provide an avenue for therapeutic intervention. 1,25(OH)2D3-induced tolerogenic DC are in clinical trial for MS. We show that the protocol of in vitro DC differentiation qualitatively and quantitatively affects secretion of cytokines and chemokines deeply involved in MS pathogenesis.
Keywords: chemokine; cytokine; immune pathophysiology; modifiable environmental factor; monocyte-derived dendritic cell; multiple sclerosis; type I IFN; vitamin D.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
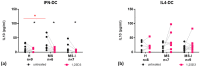
Similar articles
-
CCL2 induction by 1,25(OH)2D3 in dendritic cells from healthy donors and multiple sclerosis patients.J Steroid Biochem Mol Biol. 2014 Oct;144 Pt A:102-5. doi: 10.1016/j.jsbmb.2013.10.018. Epub 2013 Nov 1. J Steroid Biochem Mol Biol. 2014. PMID: 24184699
-
Immunomodulatory Effects of 1,25-Dihydroxyvitamin D3 on Dendritic Cells Promote Induction of T Cell Hyporesponsiveness to Myelin-Derived Antigens.J Immunol Res. 2016;2016:5392623. doi: 10.1155/2016/5392623. Epub 2016 Sep 14. J Immunol Res. 2016. PMID: 27703987 Free PMC article.
-
Effects of Vitamin D and Dexamethasone on Lymphocyte Proportions and Their Associations With Serum Concentrations of 25-Hydroxyvitamin D3In Vitro in Patients With Multiple Sclerosis or Neuromyelitis Optica Spectrum Disorder.Front Immunol. 2021 Jul 29;12:677041. doi: 10.3389/fimmu.2021.677041. eCollection 2021. Front Immunol. 2021. PMID: 34394078 Free PMC article.
-
The Role of Vitamin D in Neuroprotection in Multiple Sclerosis: An Update.Nutrients. 2023 Jun 30;15(13):2978. doi: 10.3390/nu15132978. Nutrients. 2023. PMID: 37447304 Free PMC article. Review.
-
1α,25-Dihydroxyvitamin D3 and its analogs as modulators of human dendritic cells: a comparison dose-titration study.J Steroid Biochem Mol Biol. 2013 Jul;136:160-5. doi: 10.1016/j.jsbmb.2012.10.009. Epub 2012 Oct 23. J Steroid Biochem Mol Biol. 2013. PMID: 23098690 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

