Sevoflurane Induces a Cyclophilin D-Dependent Decrease of Neural Progenitor Cells Migration
- PMID: 37047719
- PMCID: PMC10095407
- DOI: 10.3390/ijms24076746
Sevoflurane Induces a Cyclophilin D-Dependent Decrease of Neural Progenitor Cells Migration
Abstract
Clinical studies have suggested that repeated exposure to anesthesia and surgery at a young age may increase the risk of cognitive impairment. Our previous research has shown that sevoflurane can affect neurogenesis and cognitive function in young animals by altering cyclophilin D (CypD) levels and mitochondrial function. Neural progenitor cells (NPCs) migration is associated with cognitive function in developing brains. However, it is unclear whether sevoflurane can regulate NPCs migration via changes in CypD. To address this question, we treated NPCs harvested from wild-type (WT) and CypD knockout (KO) mice and young WT and CypD KO mice with sevoflurane. We used immunofluorescence staining, wound healing assay, transwell assay, mass spectrometry, and Western blot to assess the effects of sevoflurane on CypD, reactive oxygen species (ROS), doublecortin levels, and NPCs migration. We showed that sevoflurane increased levels of CypD and ROS, decreased levels of doublecortin, and reduced migration of NPCs harvested from WT mice in vitro and in WT young mice. KO of CypD attenuated these effects, suggesting that a sevoflurane-induced decrease in NPCs migration is dependent on CypD. Our findings have established a system for future studies aimed at exploring the impacts of sevoflurane anesthesia on the impairment of NPCs migration.
Keywords: cyclophilin D; doublecortin; migration; neural progenitor cells; sevoflurane.
Conflict of interest statement
The authors declare no conflict interest.
Figures
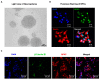
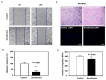
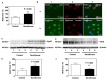




Similar articles
-
Cyclophilin D Contributes to Anesthesia Neurotoxicity in the Developing Brain.Front Cell Dev Biol. 2020 Feb 11;7:396. doi: 10.3389/fcell.2019.00396. eCollection 2019. Front Cell Dev Biol. 2020. PMID: 32117955 Free PMC article.
-
Melatonin attenuates sevoflurane-induced hippocampal damage and cognitive deficits in neonatal mice by suppressing CypD in parvalbumin neurons.Brain Res Bull. 2023 Nov;204:110809. doi: 10.1016/j.brainresbull.2023.110809. Epub 2023 Nov 4. Brain Res Bull. 2023. PMID: 37931809
-
Mitochondrial cyclophilin-D as a critical mediator of ischaemic preconditioning.Cardiovasc Res. 2010 Oct 1;88(1):67-74. doi: 10.1093/cvr/cvq113. Epub 2010 Apr 16. Cardiovasc Res. 2010. PMID: 20400621 Free PMC article.
-
Cyclophilin D Modulates the Cardiac Mitochondrial Target of Isoflurane, Sevoflurane, and Desflurane.J Cardiovasc Pharmacol. 2017 May;69(5):326-334. doi: 10.1097/FJC.0000000000000479. J Cardiovasc Pharmacol. 2017. PMID: 28328748
-
Small-molecule inhibitors of cyclophilin D as potential therapeutics in mitochondria-related diseases.Med Res Rev. 2022 Sep;42(5):1822-1855. doi: 10.1002/med.21892. Epub 2022 May 16. Med Res Rev. 2022. PMID: 35575048 Review.
Cited by
-
Nissl Granules, Axonal Regeneration, and Regenerative Therapeutics: A Comprehensive Review.Cureus. 2023 Oct 28;15(10):e47872. doi: 10.7759/cureus.47872. eCollection 2023 Oct. Cureus. 2023. PMID: 38022048 Free PMC article. Review.
-
Inhalation Anesthetics Play a Janus-Faced Role in Self-Renewal and Differentiation of Stem Cells.Biomolecules. 2024 Sep 18;14(9):1167. doi: 10.3390/biom14091167. Biomolecules. 2024. PMID: 39334933 Free PMC article. Review.
-
Unraveling the role and mechanism of mitochondria in postoperative cognitive dysfunction: a narrative review.J Neuroinflammation. 2024 Nov 12;21(1):293. doi: 10.1186/s12974-024-03285-3. J Neuroinflammation. 2024. PMID: 39533332 Free PMC article. Review.
-
Special Issue "Stem Cell Biology & Regenerative Medicine".Int J Mol Sci. 2023 Aug 16;24(16):12855. doi: 10.3390/ijms241612855. Int J Mol Sci. 2023. PMID: 37629035 Free PMC article.
References
-
- Wilder R.T., Flick R.P., Sprung J., Katusic S.K., Barbaresi W.J., Mickelson C., Gleich S.J., Schroeder D.R., Weaver A.L., Warner D.O. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. - DOI - PMC - PubMed
-
- Sun L.S., Li G., Miller T.L., Salorio C., Byrne M.W., Bellinger D.C., Ing C., Park R., Radcliffe J., Hays S.R., et al. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. JAMA. 2016;315:2312–2320. doi: 10.1001/jama.2016.6967. - DOI - PMC - PubMed
-
- Davidson A.J., Disma N., de Graaff J.C., Withington D.E., Dorris L., Bell G., Stargatt R., Bellinger D.C., Schuster T., Arnup S.J., et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): An international multicentre, randomised controlled trial. Lancet. 2016;387:239–250. doi: 10.1016/S0140-6736(15)00608-X. - DOI - PMC - PubMed
-
- Warner D.O., Chelonis J.J., Paule M.G., Frank R.D., Lee M., Zaccariello M.J., Katusic S.K., Schroeder D.R., Hanson A.C., Schulte P.J., et al. Performance on the Operant Test Battery in young children exposed to procedures requiring general anaesthesia: The MASK study. Br. J. Anaesth. 2019;122:470–479. doi: 10.1016/j.bja.2018.12.020. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

