Unraveling Structural Alerts in Marketed Drugs for Improving Adverse Outcome Pathway Framework of Drug-Induced QT Prolongation
- PMID: 37047744
- PMCID: PMC10095420
- DOI: 10.3390/ijms24076771
Unraveling Structural Alerts in Marketed Drugs for Improving Adverse Outcome Pathway Framework of Drug-Induced QT Prolongation
Abstract
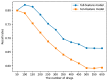
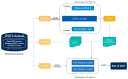
In pharmaceutical treatment, many non-cardiac drugs carry the risk of prolonging the QT interval, which can lead to fatal cardiac complications such as torsades de points (TdP). Although the unexpected blockade of ion channels has been widely considered to be one of the main reasons for affecting the repolarization phase of the cardiac action potential and leading to QT interval prolongation, the lack of knowledge regarding chemical structures in drugs that may induce the prolongation of the QT interval remains a barrier to further understanding the underlying mechanism and developing an effective prediction strategy. In this study, we thoroughly investigated the differences in chemical structures between QT-prolonging drugs and drugs with no drug-induced QT prolongation (DIQT) concerns, based on the Drug-Induced QT Prolongation Atlas (DIQTA) dataset. Three categories of structural alerts (SAs), namely amines, ethers, and aromatic compounds, appeared in large quantities in QT-prolonging drugs, but rarely in drugs with no DIQT concerns, indicating a close association between SAs and the risk of DIQT. Moreover, using the molecular descriptors associated with these three categories of SAs as features, the structure-activity relationship (SAR) model for predicting the high risk of inducing QT interval prolongation of marketed drugs achieved recall rates of 72.5% and 80.0% for the DIQTA dataset and the FDA Adverse Event Reporting System (FAERS) dataset, respectively. Our findings may promote a better understanding of the mechanism of DIQT and facilitate research on cardiac adverse drug reactions in drug development.
Keywords: adverse outcome pathway; drug safety evaluation; drug-induced prolongation of QT interval; machine learning; structure–activity relationship.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Drug-induced QT Prolongation Atlas (DIQTA) for enhancing cardiotoxicity management.Drug Discov Today. 2022 Mar;27(3):831-837. doi: 10.1016/j.drudis.2021.10.009. Epub 2021 Oct 27. Drug Discov Today. 2022. PMID: 34718206 Review.
-
Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development.Cardiovasc Res. 2003 Apr 1;58(1):32-45. doi: 10.1016/s0008-6363(02)00846-5. Cardiovasc Res. 2003. PMID: 12667944 Review.
-
[Drug induced QT prolongation].Wien Klin Wochenschr. 2008;120(5-6):128-35. doi: 10.1007/s00508-008-0940-6. Wien Klin Wochenschr. 2008. PMID: 18365152 Review. German.
-
Molecular predictors of drug-induced prolongation of the QT interval.Curr Med Chem Cardiovasc Hematol Agents. 2005 Apr;3(2):105-18. doi: 10.2174/1568016053544318. Curr Med Chem Cardiovasc Hematol Agents. 2005. PMID: 15853698 Review.
-
Drug-induced QT prolongation and proarrhythmia: an inevitable link?Europace. 2007 Sep;9 Suppl 4:iv16-22. doi: 10.1093/europace/eum167. Europace. 2007. PMID: 17766320 Review.
Cited by
-
Advancing Adverse Drug Reaction Prediction with Deep Chemical Language Model for Drug Safety Evaluation.Int J Mol Sci. 2024 Apr 20;25(8):4516. doi: 10.3390/ijms25084516. Int J Mol Sci. 2024. PMID: 38674100 Free PMC article.
-
Evaluation of the Effect of Momelotinib on Cardiac Repolarization: A Thorough QT Study.Clin Pharmacol Drug Dev. 2025 Apr;14(4):333-342. doi: 10.1002/cpdd.1509. Epub 2025 Jan 23. Clin Pharmacol Drug Dev. 2025. PMID: 39844679 Free PMC article. Clinical Trial.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

