The Immunological Profile of SARS-CoV-2 Infection in Children Is Linked to Clinical Severity and Age
- PMID: 37047752
- PMCID: PMC10095251
- DOI: 10.3390/ijms24076779
The Immunological Profile of SARS-CoV-2 Infection in Children Is Linked to Clinical Severity and Age
Abstract
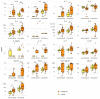
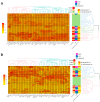
Coronavirus disease 19 (COVID-19) is clinically less severe in children, even if the wide variety and degree of severity of symptoms reported in children pose a still-unresolved challenge for clinicians. We performed an in-depth analysis of the immunological profiles of 18 hospitalized SARS-CoV-2-infected children, whose results were compared to those obtained from 13 age- and sex-matched healthy controls (HC). The patients were categorized as paucisymptomatic/moderate (55.6%) or severe/critical (44.5%) according to established diagnostic criteria and further stratified into the categories of infants (1-12 months), children (1-12 years), and adolescents (>12 years). We assessed SARS-CoV-2-specific RBD antibodies (Ab), neutralizing antibodies (nAb), and circulating cytokines/chemokines in the plasma, and the SARS-CoV-2-specific immune response was measured in PBMCs by gene expression and secretome analyses. Our results showed peculiar circulating cytokine/chemokine profiles among patients sharing a similar clinical phenotype. A cluster of patients consisting of infants with severe symptoms presented hyperinflammatory profiles, together with extremely polarized antibody profiles. In a second cluster consisting of paucisymptomatic patients, a less pronounced increase in the level of inflammatory cytokines, together with an association between the selected cytokines and humoral responses, was observed. A third cluster, again consisting of paucisymptomatic patients, showed a circulating cytokine/chemokine profile which overlapped with that of the HC. The SARS-CoV-2-stimulated production of pro-inflammatory proteins, T lymphocyte activation, and migration-specific proteins, were significantly increased in SARS-CoV-2-infected children compared to the HC. Our findings suggest that immune response activation in the course of SARS-CoV-2 infection in children is directly correlated with clinical severity and, to a lesser extent, age.
Keywords: COVID-19; SARS-CoV-2 infection; children; immune response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Viner R.M., Mytton O.T., Bonell C., Melendez-Torres G.J., Ward J., Hudson L., Waddington C., Thomas J., Russell S., van der Klis F., et al. Susceptibility to SARS-CoV-2 Infection Among Children and Adolescents Compared with Adults: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2021;175:143–156. doi: 10.1001/jamapediatrics.2020.4573. - DOI - PMC - PubMed
-
- Sharma C., Ganigara M., Galeotti C., Burns J., Berganza F.M., Hayes D.A., Singh-Grewal D., Bharath S., Sajjan S., Bayry J. Multisystem Inflammatory Syndrome in Children and Kawasaki Disease: A Critical Comparison. Nat. Rev. Rheumatol. 2021;17:731–748. doi: 10.1038/s41584-021-00709-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

