MC-PRPA-HLFIA Cascade Detection System for Point-of-Care Testing Pan-Drug-Resistant Genes in Urinary Tract Infection Samples
- PMID: 37047757
- PMCID: PMC10095522
- DOI: 10.3390/ijms24076784
MC-PRPA-HLFIA Cascade Detection System for Point-of-Care Testing Pan-Drug-Resistant Genes in Urinary Tract Infection Samples
Abstract
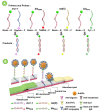
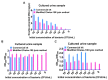
Recently, urinary tract infection (UTI) triggered by bacteria carrying pan-drug-resistant genes, including carbapenem resistance gene blaNDM and blaKPC, colistin resistance gene mcr-1, and tet(X) for tigecycline resistance, have been reported, posing a serious challenge to the treatment of clinical UTI. Therefore, point-of-care (POC) detection of these genes in UTI samples without the need for pre-culturing is urgently needed. Based on PEG 200-enhanced recombinase polymerase amplification (RPA) and a refined Chelex-100 lysis method with HRP-catalyzed lateral flow immunoassay (LFIA), we developed an MCL-PRPA-HLFIA cascade assay system for detecting these genes in UTI samples. The refined Chelex-100 lysis method extracts target DNA from UTI samples in 20 min without high-speed centrifugation or pre-incubation of urine samples. Following optimization, the cascade detection system achieved an LOD of 102 CFU/mL with satisfactory specificity and could detect these genes in both simulated and actual UTI samples. It takes less than an hour to complete the process without the use of high-speed centrifuges or other specialized equipment, such as PCR amplifiers. The MCL-PRPA-HLFIA cascade assay system provides new ideas for the construction of rapid detection methods for pan-drug-resistant genes in clinical UTI samples and provides the necessary medication guidance for UTI treatment.
Keywords: Chelex-100; extensively drug-resistant genes; lateral flow immunoassay; recombinase polymerase amplification; urinary tract infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Rapid On-Site Detection of Extensively Drug-Resistant Genes in Enterobacteriaceae via Enhanced Recombinase Polymerase Amplification and Lateral Flow Biosensor.Microbiol Spectr. 2022 Dec 21;10(6):e0334422. doi: 10.1128/spectrum.03344-22. Epub 2022 Nov 29. Microbiol Spectr. 2022. PMID: 36445091 Free PMC article.
-
Colistin- and Carbapenem-Resistant Escherichia coli Harboring mcr-1 and blaNDM-5, Causing a Complicated Urinary Tract Infection in a Patient from the United States.mBio. 2016 Aug 30;7(4):e01191-16. doi: 10.1128/mBio.01191-16. mBio. 2016. PMID: 27578755 Free PMC article.
-
Development of a panel of recombinase polymerase amplification assays for detection of common bacterial urinary tract infection pathogens.J Appl Microbiol. 2017 Aug;123(2):544-555. doi: 10.1111/jam.13493. J Appl Microbiol. 2017. PMID: 28510991 Free PMC article.
-
Urinary Tract Infections Detection with Molecular Biomarkers.Biomolecules. 2024 Nov 30;14(12):1540. doi: 10.3390/biom14121540. Biomolecules. 2024. PMID: 39766247 Free PMC article. Review.
-
Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli.Dan Med Bull. 2011 Apr;58(4):B4187. Dan Med Bull. 2011. PMID: 21466767 Review.
Cited by
-
Analysis of urine cell-free DNA in bladder cancer diagnosis by emerging bioactive technologies and materials.Front Bioeng Biotechnol. 2024 Sep 4;12:1458362. doi: 10.3389/fbioe.2024.1458362. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39295845 Free PMC article. Review.
References
-
- Cai T., Nesi G., Mazzoli S., Meacci F., Lanzafame P., Caciagli P., Mereu L., Tateo S., Malossini G., Selli C., et al. Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infections. Clin. Infect. Dis. 2015;61:1655–1661. doi: 10.1093/cid/civ696. - DOI - PubMed
-
- Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. - DOI - PubMed
-
- Liu D., Zhai W., Song H., Fu Y., Schwarz S., He T., Bai L., Wang Y., Walsh T.R., Shen J. Identification of the novel tigecycline resistance gene tet(X6) and its variants in Myroides, Acinetobacter and Proteus of food animal origin. J. Antimicrob. Chemother. 2020;75:1428–1431. doi: 10.1093/jac/dkaa037. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

