Mixed-Linkage Glucan Is the Main Carbohydrate Source and Starch Is an Alternative Source during Brachypodium Grain Germination
- PMID: 37047802
- PMCID: PMC10095428
- DOI: 10.3390/ijms24076821
Mixed-Linkage Glucan Is the Main Carbohydrate Source and Starch Is an Alternative Source during Brachypodium Grain Germination
Abstract
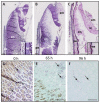
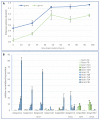
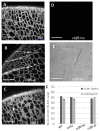
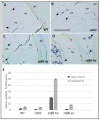
Seeds of the model grass Brachypodium distachyon are unusual because they contain very little starch and high levels of mixed-linkage glucan (MLG) accumulated in thick cell walls. It was suggested that MLG might supplement starch as a storage carbohydrate and may be mobilised during germination. In this work, we observed massive degradation of MLG during germination in both endosperm and nucellar epidermis. The enzymes responsible for the MLG degradation were identified in germinated grains and characterized using heterologous expression. By using mutants targeting MLG biosynthesis genes, we showed that the expression level of genes coding for MLG and starch-degrading enzymes was modified in the germinated grains of knocked-out cslf6 mutants depleted in MLG but with higher starch content. Our results suggest a substrate-dependent regulation of the storage sugars during germination. These overall results demonstrated the function of MLG as the main carbohydrate source during germination of Brachypodium grain. More astonishingly, cslf6 Brachypodium mutants are able to adapt their metabolism to the lack of MLG by modifying the energy source for germination and the expression of genes dedicated for its use.
Keywords: Brachypodium; cell wall; germination; grain; lichenase; mixed-linkage glucan (MLG).
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Disruption of Brachypodium lichenase alters metabolism of mixed-linkage glucan and starch.Plant J. 2022 Feb;109(4):927-939. doi: 10.1111/tpj.15603. Epub 2021 Dec 17. Plant J. 2022. PMID: 34845787
-
Characterisation of Cellulose Synthase Like F6 (CslF6) Mutants Shows Altered Carbon Metabolism in β-D-(1,3;1,4)-Glucan Deficient Grain in Brachypodium distachyon.Front Plant Sci. 2021 Jan 11;11:602850. doi: 10.3389/fpls.2020.602850. eCollection 2020. Front Plant Sci. 2021. PMID: 33505412 Free PMC article.
-
In the grass species Brachypodium distachyon, the production of mixed-linkage (1,3;1,4)-β-glucan (MLG) occurs in the Golgi apparatus.Plant J. 2018 Mar;93(6):1062-1075. doi: 10.1111/tpj.13830. Epub 2018 Mar 6. Plant J. 2018. PMID: 29377449
-
Iminosugar inhibitors of carbohydrate-active enzymes that underpin cereal grain germination and endosperm metabolism.Biochem Soc Trans. 2016 Feb;44(1):159-65. doi: 10.1042/BST20150222. Biochem Soc Trans. 2016. PMID: 26862201 Free PMC article. Review.
-
Advances in Cell Wall Matrix Research with a Focus on Mixed-Linkage Glucan.Plant Cell Physiol. 2021 Dec 27;62(12):1839-1846. doi: 10.1093/pcp/pcab106. Plant Cell Physiol. 2021. PMID: 34245308 Review.
Cited by
-
Genetic Approaches to Increase Arabinoxylan and β-Glucan Content in Wheat.Plants (Basel). 2023 Sep 8;12(18):3216. doi: 10.3390/plants12183216. Plants (Basel). 2023. PMID: 37765380 Free PMC article. Review.
-
Is the CslF6 gene involved in the accumulation of (1,3;1,4)-β-D-glucan in wheats, their wild relatives and their hybrids?Food Chem (Oxf). 2024 Jul 5;9:100212. doi: 10.1016/j.fochms.2024.100212. eCollection 2024 Dec 30. Food Chem (Oxf). 2024. PMID: 39679358 Free PMC article.
-
The plant cell wall-dynamic, strong, and adaptable-is a natural shapeshifter.Plant Cell. 2024 May 1;36(5):1257-1311. doi: 10.1093/plcell/koad325. Plant Cell. 2024. PMID: 38301734 Free PMC article. Review.
-
The knockout of ClaCSLH1 induced dwarfing in watermelon.Theor Appl Genet. 2025 May 19;138(6):120. doi: 10.1007/s00122-025-04909-9. Theor Appl Genet. 2025. PMID: 40387943
References
-
- Trafford K., Haleux P., Henderson M., Parker M., Shirley N.J., Tucker M.R., Fincher G.B., Burton R.A. Grain development in Brachypodium and other grasses: Possible interactions between cell expansion, starch deposition, and cell-wall synthesis. J. Exp. Bot. 2013;64:5033–5047. doi: 10.1093/jxb/ert292. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

