The Geroprotective Drug Candidate CMS121 Alleviates Diabetes, Liver Inflammation, and Renal Damage in db/db Leptin Receptor Deficient Mice
- PMID: 37047807
- PMCID: PMC10095029
- DOI: 10.3390/ijms24076828
The Geroprotective Drug Candidate CMS121 Alleviates Diabetes, Liver Inflammation, and Renal Damage in db/db Leptin Receptor Deficient Mice
Abstract
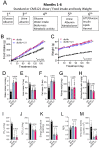
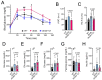
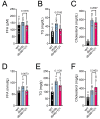
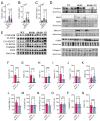
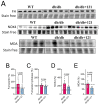
db/db mice, which lack leptin receptors and exhibit hyperphagia, show disturbances in energy metabolism and are a model of obesity and type 2 diabetes. The geroneuroprotector drug candidate CMS121 has been shown to be effective in animal models of Alzheimer's disease and aging through the modulation of metabolism. Thus, the hypothesis was that CMS121 could protect db/db mice from metabolic defects and thereby reduce liver inflammation and kidney damage. The mice were treated with CMS121 in their diet for 6 months. No changes were observed in food and oxygen consumption, body mass, or locomotor activity compared to control db/db mice, but a 5% reduction in body weight was noted. Improved glucose tolerance and reduced HbA1c and insulin levels were also seen. Blood and liver triglycerides and free fatty acids decreased. Improved metabolism was supported by lower levels of fatty acid metabolites in the urine. Markers of liver inflammation, including NF-κB, IL-18, caspase 3, and C reactive protein, were lowered by the CMS121 treatment. Urine markers of kidney damage were improved, as evidenced by lower urinary levels of NGAL, clusterin, and albumin. Urine metabolomics studies provided further evidence for kidney protection. Mitochondrial protein markers were elevated in db/db mice, but CMS121 restored the renal levels of NDUFB8, UQCRC2, and VDAC. Overall, long-term CMS121 treatment alleviated metabolic imbalances, liver inflammation, and reduced markers of kidney damage. Thus, this study provides promising evidence for the potential therapeutic use of CMS121 in treating metabolic disorders.
Keywords: Alzheimer’s disease; geroneuroprotector; inflammation; kidney damage; metabolic disorders; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
CMS121: a novel approach to mitigate aging-related obesity and metabolic dysfunction.Aging (Albany NY). 2024 Mar 20;16(6):4980-4999. doi: 10.18632/aging.205673. Epub 2024 Mar 20. Aging (Albany NY). 2024. PMID: 38517358 Free PMC article.
-
Bilirubin increases insulin sensitivity in leptin-receptor deficient and diet-induced obese mice through suppression of ER stress and chronic inflammation.Endocrinology. 2014 Mar;155(3):818-28. doi: 10.1210/en.2013-1667. Epub 2014 Jan 1. Endocrinology. 2014. PMID: 24424052 Free PMC article.
-
Timed Inhibition of Orexin System by Suvorexant Improved Sleep and Glucose Metabolism in Type 2 Diabetic db/db Mice.Endocrinology. 2016 Nov;157(11):4146-4157. doi: 10.1210/en.2016-1404. Epub 2016 Sep 15. Endocrinology. 2016. PMID: 27631554
-
Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes.Curr Diabetes Rev. 2014 Mar;10(2):131-45. doi: 10.2174/1573399810666140508121012. Curr Diabetes Rev. 2014. PMID: 24809394 Free PMC article. Review.
-
[The research applications of db/db mouse].Sheng Li Ke Xue Jin Zhan. 2013 Feb;44(1):12-8. Sheng Li Ke Xue Jin Zhan. 2013. PMID: 23671994 Review. Chinese.
Cited by
-
ACACA reduces lipid accumulation through dual regulation of lipid metabolism and mitochondrial function via AMPK- PPARα- CPT1A axis.J Transl Med. 2024 Feb 23;22(1):196. doi: 10.1186/s12967-024-04942-0. J Transl Med. 2024. PMID: 38395901 Free PMC article.
-
Fecal Microbiome and Urine Metabolome Profiling of Type 2 Diabetes.J Microbiol Biotechnol. 2025 Mar 11;35:e2411071. doi: 10.4014/jmb.2411.11071. J Microbiol Biotechnol. 2025. PMID: 40147938 Free PMC article.
-
Effect and underlying mechanism of Huangjing Qianshi decoction in pre-diabetes mouse model.BMC Complement Med Ther. 2025 Apr 23;25(1):151. doi: 10.1186/s12906-025-04893-z. BMC Complement Med Ther. 2025. PMID: 40269867 Free PMC article.
-
CMS121: a novel approach to mitigate aging-related obesity and metabolic dysfunction.Aging (Albany NY). 2024 Mar 20;16(6):4980-4999. doi: 10.18632/aging.205673. Epub 2024 Mar 20. Aging (Albany NY). 2024. PMID: 38517358 Free PMC article.
-
Elucidating the function of clusterin in the progression of diabetic kidney disease.Front Pharmacol. 2025 May 14;16:1573654. doi: 10.3389/fphar.2025.1573654. eCollection 2025. Front Pharmacol. 2025. PMID: 40438587 Free PMC article. Review.
References
-
- WHO World Health Organization Screening for Type 2 Diabetes: Report of a World Health Organization and International Diabetes Federation Meeting. [(accessed on 5 January 2023)];2003 Available online: http://www.who.int/diabetes/publications/en/screening_mnc03.pdf.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

