Quality-Adjusted Life Years in Erythropoietic Protoporphyria and Other Rare Diseases: A Patient-Initiated EQ-5D Feasibility Study
- PMID: 37047912
- PMCID: PMC10094018
- DOI: 10.3390/ijerph20075296
Quality-Adjusted Life Years in Erythropoietic Protoporphyria and Other Rare Diseases: A Patient-Initiated EQ-5D Feasibility Study
Abstract
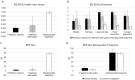
Erythropoietic protoporphyria (EPP) is an ultra-rare inborn error of metabolism characterised by painful phototoxic burn injuries after short exposure times to visible light. Patients with EPP are highly adapted to their condition which makes the quantification of their health-related quality of life (QoL) challenging. In the presented patient-initiated feasibility study, we describe a new approach to assess treatment benefits in EPP by measuring QoL with the generic EQ-5D instrument in five patients under long-term (≥two years) treatment with afamelanotide, the first approved therapy for EPP. For the study, we selected patients with EPP who in addition were affected by an involuntary treatment interruption (caused by a temporary reimbursement suspension) because we hypothesized that individuals who had previously unlearned their adaptation are better able to assess their life without treatment than treatment-naïve patients. QoL under treatment was comparable to the age-matched population norm, and retrospective results for a treatment interruption and phototoxic reaction time point were comparable to the QoL of patients with chronic neuropathic pain and acute burn injuries, respectively. The results were accepted by the National Institute for Health and Care Excellence in England for their evaluation of the cost-effectiveness of afamelanotide, i.e., the calculation of quality-adjusted life years.
Keywords: EQ-5D; National Institute for Health and Care Excellence; afamelanotide; erythropoietic protoporphyria; health technology assessment; highly specialised technologies; orphan drug; quality of life; quality-adjusted life years.
Conflict of interest statement
J.B.-A., F.G., F.B., M.P., C.D., M.H.A., and R.F. declare that they do not have any conflicts of interest. AM had an unrestricted research grant from Clinuvel, the manufacturer of afamelanotide, and currently receives financial support for a porphyria study nurse from Alnylam. Both grants were paid to the Stiftung für wissenschaftliche Forschung am Stadtspital Zürich.
Figures
Similar articles
-
Increased phototoxic burn tolerance time and quality of life in patients with erythropoietic protoporphyria treated with afamelanotide - a three years observational study.Orphanet J Rare Dis. 2020 Aug 18;15(1):213. doi: 10.1186/s13023-020-01505-6. Orphanet J Rare Dis. 2020. PMID: 32811524 Free PMC article.
-
Afamelanotide for prevention of phototoxicity in erythropoietic protoporphyria.Expert Rev Clin Pharmacol. 2021 Feb;14(2):151-160. doi: 10.1080/17512433.2021.1879638. Expert Rev Clin Pharmacol. 2021. PMID: 33507118 Review.
-
Erythropoietic protoporphyria in the Netherlands: Clinical features, psychosocial impact and the effect of afamelanotide.J Dermatol. 2023 Apr;50(4):445-452. doi: 10.1111/1346-8138.16690. Epub 2022 Dec 28. J Dermatol. 2023. PMID: 36579412
-
Association of Afamelanotide With Improved Outcomes in Patients With Erythropoietic Protoporphyria in Clinical Practice.JAMA Dermatol. 2020 May 1;156(5):570-575. doi: 10.1001/jamadermatol.2020.0352. JAMA Dermatol. 2020. PMID: 32186677 Free PMC article.
-
Afamelanotide: A Review in Erythropoietic Protoporphyria.Am J Clin Dermatol. 2016 Apr;17(2):179-85. doi: 10.1007/s40257-016-0184-6. Am J Clin Dermatol. 2016. PMID: 26979527 Review.
Cited by
-
Fair Funding Decisions: Consistency of the Time Horizons Used in the Calculation of Quality-Adjusted Life Years for Therapies for Very Rare Diseases by the National Institute for Health and Care Excellence in England.Int J Environ Res Public Health. 2024 May 13;21(5):616. doi: 10.3390/ijerph21050616. Int J Environ Res Public Health. 2024. PMID: 38791830 Free PMC article.
-
Development and Content Validation of Novel Patient-Reported Outcome Measures to Assess Disease Severity and Change in Patients with Erythropoietic Protoporphyria: The EPP Impact Questionnaire (EPIQ).Patient Relat Outcome Meas. 2024 Feb 14;15:17-30. doi: 10.2147/PROM.S438892. eCollection 2024. Patient Relat Outcome Meas. 2024. PMID: 38375415 Free PMC article.
-
Comparison of the measurement properties and consistency between the EQ-5D-3L and EQ-5D-Y-3L in adolescents aged 15-17 in China.Health Qual Life Outcomes. 2024 Jul 29;22(1):59. doi: 10.1186/s12955-024-02275-6. Health Qual Life Outcomes. 2024. PMID: 39075537 Free PMC article.
References
-
- Whatley S.D., Ducamp S., Gouya L., Grandchamp B., Beaumont C., Badminton M.N., Elder G.H., Holme S.A., Anstey A.V., Parker M., et al. C-Terminal Deletions in the ALAS2 Gene Lead to Gain of Function and Cause X-Linked Dominant Protoporphyria without Anemia or Iron Overload. Am. J. Hum. Genet. 2008;83:408–414. doi: 10.1016/j.ajhg.2008.08.003. - DOI - PMC - PubMed
-
- Yien Y.Y., Ducamp S., van der Vorm L.N., Kardon J.R., Manceau H., Kannengiesser C., Bergonia H.A., Kafina M.D., Karim Z., Gouya L., et al. Mutation in Human CLPX Elevates Levels of δ-Aminolevulinate Synthase and Protoporphyrin IX to Promote Erythropoietic Protoporphyria. Proc. Natl. Acad. Sci. USA. 2017;114:E8045. doi: 10.1073/pnas.1700632114. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

