Rotating Magnetic Field Mitigates Ankylosing Spondylitis Targeting Osteocytes and Chondrocytes via Ameliorating Immune Dysfunctions
- PMID: 37048045
- PMCID: PMC10093245
- DOI: 10.3390/cells12070972
Rotating Magnetic Field Mitigates Ankylosing Spondylitis Targeting Osteocytes and Chondrocytes via Ameliorating Immune Dysfunctions
Abstract
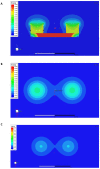
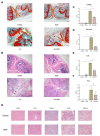
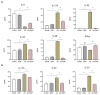
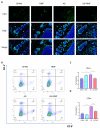
Ankylosing spondylitis (AS) is clinically characterized by bone fusion that is induced by the pathological formation of extra bone. Unfortunately, the fundamental mechanism and related therapies remain unclear. The loss of SHP-2 (encoded by Ptpn11) in CD4-Cre;Ptpn11f/f mice resulted in the induction of AS-like pathological characteristics, including spontaneous cartilage and bone lesions, kyphosis, and arthritis. Hence, this mouse was utilized as an AS model in this study. As one of the basic physical fields, the magnetic field (MF) has been proven to be an effective treatment method for articular cartilage degeneration. In this study, the effects of a rotating magnetic field (RMF; 0.2 T, 4 Hz) on an AS-like mouse model were investigated. The RMF treatment (2 h/d, 0.2 T, 4 Hz) was performed on AS mice from two months after birth until the day before sampling. The murine specimens were subjected to transcriptomics, immunomics, and metabolomics analyses, combined with molecular and pathological experiments. The results demonstrated that the mitigation of inflammatory deterioration resulted in an increase in functional osteogenesis and a decrease in dysfunctional osteolysis due to the maintenance of bone homeostasis via the RANKL/RANK/OPG signaling pathway. Additionally, by regulating the ratio of CD4+ and CD8+ T-cells, RMF treatment rebalanced the immune microenvironment in skeletal tissue. It has been observed that RMF interventions have the potential to alleviate AS, including by decreasing pathogenicity and preventing disease initiation. Consequently, RMF, as a moderately physical therapeutic strategy, could be considered to alleviate the degradation of cartilage and bone tissue in AS and as a potential option to halt the progression of AS.
Keywords: CD4-CKO mice; ankylosing spondylitis; cartilage tissues; inflammation; rotating magnetic field.
Conflict of interest statement
The authors declare no conflict of interest. They confirm that this manuscript has not been published previously. It is not under consideration for publication elsewhere, and if accepted, it will not be published elsewhere in the same form, in English or in any other language. The corresponding author confirms the above statements on behalf of the co-authors.
Figures




Similar articles
-
Targeting chondrocytes for arresting bony fusion in ankylosing spondylitis.Nat Commun. 2021 Nov 11;12(1):6540. doi: 10.1038/s41467-021-26750-6. Nat Commun. 2021. PMID: 34764263 Free PMC article.
-
Purine metabolites promote ectopic new bone formation in ankylosing spondylitis.Int Immunopharmacol. 2023 Mar;116:109810. doi: 10.1016/j.intimp.2023.109810. Epub 2023 Feb 10. Int Immunopharmacol. 2023. PMID: 36774858
-
HLA-B27-restricted antigen presentation by human chondrocytes to CD8+ T cells: potential contribution to local immunopathologic processes in ankylosing spondylitis.Arthritis Rheum. 2009 Jun;60(6):1635-46. doi: 10.1002/art.24549. Arthritis Rheum. 2009. PMID: 19479861
-
Gene therapy methods in bone and joint disorders. Evaluation of the adeno-associated virus vector in experimental models of articular cartilage disorders, periprosthetic osteolysis and bone healing.Acta Orthop Suppl. 2007 Apr;78(325):1-64. Acta Orthop Suppl. 2007. PMID: 17427340 Review.
-
Pathological mechanism of chondrocytes and the surrounding environment during osteoarthritis of temporomandibular joint.J Cell Mol Med. 2021 Jun;25(11):4902-4911. doi: 10.1111/jcmm.16514. Epub 2021 May 5. J Cell Mol Med. 2021. PMID: 33949768 Free PMC article. Review.
Cited by
-
Rotating magnetic field improved cognitive and memory impairments in a sporadic ad model of mice by regulating microglial polarization.Geroscience. 2024 Dec;46(6):6229-6256. doi: 10.1007/s11357-024-01223-y. Epub 2024 Jun 21. Geroscience. 2024. PMID: 38904930 Free PMC article.
-
Remote actuation and on-demand activation of biomaterials pre-incorporated with physical cues for bone repair.Theranostics. 2024 Jul 16;14(11):4438-4461. doi: 10.7150/thno.97610. eCollection 2024. Theranostics. 2024. PMID: 39113795 Free PMC article. Review.
-
Gradient Rotating Magnetic Fields Impairing F-Actin-Related Gene CCDC150 to Inhibit Triple-Negative Breast Cancer Metastasis by Inactivating TGF-β1/SMAD3 Signaling Pathway.Research (Wash D C). 2024 Feb 28;7:0320. doi: 10.34133/research.0320. eCollection 2024. Research (Wash D C). 2024. PMID: 38420580 Free PMC article.
-
Unveiling the Power of Magnetic-Driven Regenerative Medicine: Bone Regeneration and Functional Reconstruction.Research (Wash D C). 2025 May 22;8:0707. doi: 10.34133/research.0707. eCollection 2025. Research (Wash D C). 2025. PMID: 40405913 Free PMC article. Review.
References
-
- Cortes A., Hadler J., Pointon J.P., Robinson P.C., Karaderi T., Leo P., Cremin K., Pryce K., Harris J., Lee S., et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat. Genet. 2013;45:730–738. doi: 10.1038/ng.2667. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
