Retinal Development in a Precocial Bird Species, the Quail (Coturnix coturnix, Linnaeus 1758)
- PMID: 37048062
- PMCID: PMC10093483
- DOI: 10.3390/cells12070989
Retinal Development in a Precocial Bird Species, the Quail (Coturnix coturnix, Linnaeus 1758)
Abstract
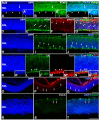
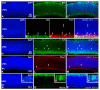
The quail (Coturnix coturnix, Linnaeus 1758), a notable model used in developmental biology, is a precocial bird species in which the processes of retinal cell differentiation and retinal histogenesis have been poorly studied. The purpose of the present research is to examine the retinogenesis in this bird species immunohistochemically and compare the results with those from previous studies in precocial and altricial birds. We found that the first PCNA-negative nuclei are detected at Stage (St) 21 in the vitreal region of the neuroblastic layer, coinciding topographically with the first αTubAc-/Tuj1-/Isl1-immunoreactive differentiating ganglion cells. At St28, the first Prox1-immunoreactive nuclei can be distinguished in the vitreal side of the neuroblastic layer (NbL), but also the first visinin-immunoreactive photoreceptors in the scleral surface. The inner plexiform layer (IPL) emerges at St32, and the outer plexiform layer (OPL) becomes visible at St35-the stage in which the first GS-immunoreactive Müller cells are distinguishable. Newly hatched animals show a well-developed stratified retina in which the PCNA-and pHisH3-immunoreactivies are absent. Therefore, retinal cell differentiation in the quail progresses in the stereotyped order conserved among vertebrates, in which ganglion cells initially appear and are followed by amacrine cells, horizontal cells, and photoreceptors. Müller glia are one of the last cell types to be born. Plexiform layers emerge following a vitreal-to-scleral gradient. Finally, our results suggest that there are no significant differences in the timing of different events involved in retinal maturation between the quail and the chicken, but the same events are delayed in an altricial bird species.
Keywords: altricial; cell differentiation; immunohistochemistry; precocial; quail; retina; retinogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Retinal differentiation in an altricial bird species, Taeniopygia guttata: An immunohistochemical study.Exp Eye Res. 2020 Jan;190:107869. doi: 10.1016/j.exer.2019.107869. Epub 2019 Nov 6. Exp Eye Res. 2020. PMID: 31705900
-
Timing and Distribution of Mitotic Activity in the Retina During Precocial and Altricial Modes of Avian Development.Front Neurosci. 2022 May 9;16:853544. doi: 10.3389/fnins.2022.853544. eCollection 2022. Front Neurosci. 2022. PMID: 35615284 Free PMC article.
-
Retinal histogenesis in an altricial avian species, the zebra finch (Taeniopygia guttata, Vieillot 1817).J Anat. 2018 Jul;233(1):106-120. doi: 10.1111/joa.12809. Epub 2018 Mar 26. J Anat. 2018. PMID: 29582431 Free PMC article.
-
Neuroendocrine and behavioral implications of endocrine disrupting chemicals in quail.Horm Behav. 2001 Sep;40(2):234-47. doi: 10.1006/hbeh.2001.1695. Horm Behav. 2001. PMID: 11534988 Review.
-
Comparative thyroid development in precocial Japanese quail and altricial ring doves.J Exp Zool Suppl. 1987;1:281-90. J Exp Zool Suppl. 1987. PMID: 3298534 Review.
References
-
- Bejarano-Escobar R., Álvarez-Hernán G., Morona R., González A., Martín-Partido G., Francisco-Morcillo J. Expression and Function of the LIM-Homeodomain Transcription Factor Islet-1 in the Developing and Mature Vertebrate Retina. Exp. Eye Res. 2015;138:22–31. doi: 10.1016/j.exer.2015.06.021. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

