Modeling the Effects of Cypermethrin Toxicity on Ovalbumin-Induced Allergic Pneumonitis Rats: Macrophage Phenotype Differentiation and p38/STAT6 Signaling Are Candidate Targets of Pirfenidone Treatment
- PMID: 37048067
- PMCID: PMC10093303
- DOI: 10.3390/cells12070994
Modeling the Effects of Cypermethrin Toxicity on Ovalbumin-Induced Allergic Pneumonitis Rats: Macrophage Phenotype Differentiation and p38/STAT6 Signaling Are Candidate Targets of Pirfenidone Treatment
Abstract
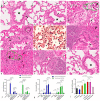
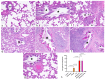
Although the classic form of asthma is characterized by chronic pneumonitis with eosinophil infiltration and steroid responsivity, asthma has multifactorial pathogenesis and various clinical phenotypes. Previous studies strongly suggested that chemical exposure could influence the severity and course of asthma and reduce its steroid responsiveness. Cypermethrin (CYP), a common pesticide used in agriculture, was investigated for the possible aggravation of the ovalbumin (OVA)-induced allergic pneumonitis and the possible induction of steroid resistance in rats. Additionally, it was investigated whether pirfenidone (PFD) could substitute dexamethasone, as an alternative treatment option, for the induced steroid resistance. Fifty-six male Wistar albino rats were randomly divided into seven groups: control, PFD alone, allergic pneumonitis, CYP alone, allergic pneumonitis/CYP-exposed, allergic pneumonitis/CYP/dexamethasone (Dex), and allergic pneumonitis/CYP/PFD-treated groups. Allergic pneumonitis was induced by three intraperitoneal OVA injections administered once a week, followed by an intranasal OVA instillation challenge. CYP (25 mg/kg/d), Dex (1 mg/kg/d), and PFD (100 mg/kg/d) were administered orally from day 15 to the end of the experiment. Bronchoalveolar lavage fluid (BALF) was analyzed for cytokine levels. Hematoxylin and eosin (H&E) and periodic acid Schiff (PAS)-stained lung sections were prepared. Immunohistochemical identification of p38 MAPK and lung macrophages was performed. The inflammatory/oxidative status of the lung and PCR-quantification of the STAT6, p38 MAPK, MUC5AC, and IL-13 genes were carried out. The allergic pneumonitis-only group showed eosinophil-mediated inflammation (p < 0.05). Further CYP exposure aggravated lung inflammation and showed steroid-resistant changes, p38 activation, neutrophil-mediated, M1 macrophage-related inflammation (p < 0.05). All changes were reversed (p < 0.05) by PFD, meanwhile not by dexamethasone treatment. Pirfenidone could replace dexamethasone treatment in the current rat model of CYP-induced severe steroid-resistant asthma via inhibiting the M1 macrophage differentiation through modulation of the STAT6/p38 MAPK pathway.
Keywords: CD86/CD206 immunohistochemistry; Th2/Th1 inflammation; asthma exacerbation; environmental triggers; p38/STAT6.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Involvements of p38 MAPK and oxidative stress in the ozone-induced enhancement of AHR and pulmonary inflammation in an allergic asthma model.Respir Res. 2017 Dec 29;18(1):216. doi: 10.1186/s12931-017-0697-4. Respir Res. 2017. PMID: 29284473 Free PMC article.
-
Comparison of asthma phenotypes in OVA-induced mice challenged via inhaled and intranasal routes.BMC Pulm Med. 2019 Dec 10;19(1):241. doi: 10.1186/s12890-019-1001-9. BMC Pulm Med. 2019. PMID: 31823765 Free PMC article.
-
Activation of p38 mitogen-activated protein kinase in ovalbumin and ozone-induced mouse model of asthma.Respirology. 2013 Nov;18 Suppl 3:20-9. doi: 10.1111/resp.12189. Respirology. 2013. PMID: 24188200
-
Where asthma and hypersensitivity pneumonitis meet and differ: noneosinophilic severe asthma.Am J Pathol. 2009 Jan;174(1):3-13. doi: 10.2353/ajpath.2009.071151. Epub 2008 Dec 12. Am J Pathol. 2009. PMID: 19074616 Free PMC article. Review.
-
The impact of formaldehyde exposure on lung inflammatory disorders: Insights into asthma, bronchitis, and pulmonary fibrosis.Chem Biol Interact. 2024 May 1;394:111002. doi: 10.1016/j.cbi.2024.111002. Epub 2024 Apr 9. Chem Biol Interact. 2024. PMID: 38604395 Review.
Cited by
-
S100A8/9 modulates perturbation and glycolysis of macrophages in allergic asthma mice.PeerJ. 2024 Apr 18;12:e17106. doi: 10.7717/peerj.17106. eCollection 2024. PeerJ. 2024. PMID: 38646478 Free PMC article.
-
Pirfenidone targeted mechanisms for alleviating methotrexate-induced testiculopathy in Wistar rats.Naunyn Schmiedebergs Arch Pharmacol. 2025 Feb;398(2):2003-2014. doi: 10.1007/s00210-024-03407-x. Epub 2024 Sep 2. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39222241
References
-
- Rahbarghazi R., Keyhanmanesh R., Aslani M.R., Hassanpour M., Ahmadi M. Bone Marrow Mesenchymal Stem Cells and Condition Media Diminish Inflammatory Adhesion Molecules of Pulmonary Endothelial Cells in an Ovalbumin-Induced Asthmatic Rat Model. Microvasc. Res. 2019;121:63–70. doi: 10.1016/j.mvr.2018.10.005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

