Derivation and Preclinical Characterization of CYT-303, a Novel NKp46-NK Cell Engager Targeting GPC3
- PMID: 37048069
- PMCID: PMC10093649
- DOI: 10.3390/cells12070996
Derivation and Preclinical Characterization of CYT-303, a Novel NKp46-NK Cell Engager Targeting GPC3
Abstract
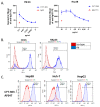
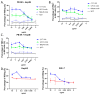
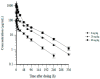
Glypican-3 (GPC3) is an oncofetal antigen that is highly expressed in multiple solid tumors, including hepatocellular carcinoma, and is barely expressed in adult normal tissues except the placenta. NKp46 activation receptor is expressed in all-natural killer (NK) cells, including tumor-infiltrating NK cells. FLEX-NKTM is a platform for the production of tetravalent multifunctional antibody NK cell engagers (NKE). CYT-303 was designed using the FLEX-NK scaffold, incorporating a novel humanized NKp46 binder that does not induce NKp46 internalization and a humanized GPC3 binder that targets the membrane-proximal lobe to mediate NK cell-redirected killing of HCC tumors. CYT-303 shows sub-nanomolar binding affinities to both GPC3 and NKp46. CYT-303 was highly potent and effective in mediating NK cell-redirected cytotoxicity against multiple HCC tumor cell lines and tumor spheroids. More interestingly, it can reverse the dysfunction induced in NK cells following repeated rounds of serial killing of tumors. It also mediated antibody-dependent cellular phagocytosis (ADCP) and complement-dependent cytotoxicity against GPC3-expressing HCC tumors. In vivo, CYT-303 showed no toxicity or cytokine release in cynomolgus monkeys up to the highest dose (60 mg/kg), administered weekly by intravenous infusion for 28 days. These results demonstrate the potential of CYT-303 to be a safe and effective therapy against HCC.
Keywords: GPC3; NK engager; NKp46; bispecific antibody; hepatocellular carcinoma; natural killer cells.
Conflict of interest statement
Antonio Arulanandam, Liang Lin, Hao-Ming Chang, Peng Gao, Armin Rath, Jean Kadouche, Daniel Teper, and Wei Li are employees and hold stock or stock options of Cytovia. Ofer Mandelboim and Armand Bensussan are consultants of Cytovia and hold stock or stock options in Cytovia.
Figures









References
-
- Birch G.C., Fehniger T.F., Romee R. Biology of NK cells and NK cells in clinic. In: Ghobadi A., DiPersio J.F., editors. Gene and Cellular Immunotherapy for Cancer. Springer International Publishing; Cham, Switzerland: 2022. pp. 293–325.
-
- Gauthier L., Morel A., Anceriz N., Rossi B., Blanchard-Alvarez A., Grondin G., Trichard S., Cesari C., Sapet M., Bosco F., et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell. 2019;177:1701–1713.e16. doi: 10.1016/j.cell.2019.04.041. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

