The Beneficial Effect of Mitochondrial Transfer Therapy in 5XFAD Mice via Liver-Serum-Brain Response
- PMID: 37048079
- PMCID: PMC10093713
- DOI: 10.3390/cells12071006
The Beneficial Effect of Mitochondrial Transfer Therapy in 5XFAD Mice via Liver-Serum-Brain Response
Abstract
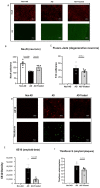
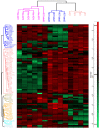
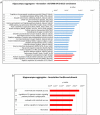
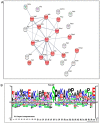
We recently reported the benefit of the IV transferring of active exogenous mitochondria in a short-term pharmacological AD (Alzheimer's disease) model. We have now explored the efficacy of mitochondrial transfer in 5XFAD transgenic mice, aiming to explore the underlying mechanism by which the IV-injected mitochondria affect the diseased brain. Mitochondrial transfer in 5XFAD ameliorated cognitive impairment, amyloid burden, and mitochondrial dysfunction. Exogenously injected mitochondria were detected in the liver but not in the brain. We detected alterations in brain proteome, implicating synapse-related processes, ubiquitination/proteasome-related processes, phagocytosis, and mitochondria-related factors, which may lead to the amelioration of disease. These changes were accompanied by proteome/metabolome alterations in the liver, including pathways of glucose, glutathione, amino acids, biogenic amines, and sphingolipids. Altered liver metabolites were also detected in the serum of the treated mice, particularly metabolites that are known to affect neurodegenerative processes, such as carnosine, putrescine, C24:1-OH sphingomyelin, and amino acids, which serve as neurotransmitters or their precursors. Our results suggest that the beneficial effect of mitochondrial transfer in the 5XFAD mice is mediated by metabolic signaling from the liver via the serum to the brain, where it induces protective effects. The high efficacy of the mitochondrial transfer may offer a novel AD therapy.
Keywords: 5XFAD; Alzheimer’s disease; amyloid; cognition; mitochondria; mitochondrial transfer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Hippocampal disruptions of synaptic and astrocyte metabolism are primary events of early amyloid pathology in the 5xFAD mouse model of Alzheimer's disease.Cell Death Dis. 2021 Oct 16;12(11):954. doi: 10.1038/s41419-021-04237-y. Cell Death Dis. 2021. PMID: 34657143 Free PMC article.
-
TDP-43 inhibitory peptide alleviates neurodegeneration and memory loss in an APP transgenic mouse model for Alzheimer's disease.Biochim Biophys Acta Mol Basis Dis. 2020 Jan 1;1866(1):165580. doi: 10.1016/j.bbadis.2019.165580. Epub 2019 Oct 31. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 31678156 Free PMC article.
-
Centella asiatica attenuates hippocampal mitochondrial dysfunction and improves memory and executive function in β-amyloid overexpressing mice.Mol Cell Neurosci. 2018 Dec;93:1-9. doi: 10.1016/j.mcn.2018.09.002. Epub 2018 Sep 22. Mol Cell Neurosci. 2018. PMID: 30253196 Free PMC article.
-
Mitochondria and Alzheimer's disease: amyloid-beta peptide uptake and degradation by the presequence protease, hPreP.J Bioenerg Biomembr. 2009 Oct;41(5):447-51. doi: 10.1007/s10863-009-9244-4. J Bioenerg Biomembr. 2009. PMID: 19798557 Review.
-
Olesoxime improves cerebral mitochondrial dysfunction and enhances Aβ levels in preclinical models of Alzheimer's disease.Exp Neurol. 2020 Jul;329:113286. doi: 10.1016/j.expneurol.2020.113286. Epub 2020 Mar 18. Exp Neurol. 2020. PMID: 32199815 Review.
Cited by
-
Mitovesicles secreted into the extracellular space of brains with mitochondrial dysfunction impair synaptic plasticity.Mol Neurodegener. 2024 Apr 14;19(1):34. doi: 10.1186/s13024-024-00721-z. Mol Neurodegener. 2024. PMID: 38616258 Free PMC article.
-
Mitochondrial Transplantation: A Novel Therapeutic Approach for Treating Diseases.MedComm (2020). 2025 Jun 11;6(6):e70253. doi: 10.1002/mco2.70253. eCollection 2025 Jun. MedComm (2020). 2025. PMID: 40502813 Free PMC article. Review.
-
Splenomegaly, Spleen Amyloidosis and Neutrophil Infiltration are Present in 3xTg-AD, but not Tg-SwDI Mice.Neuromolecular Med. 2025 Sep 4;27(1):61. doi: 10.1007/s12017-025-08884-8. Neuromolecular Med. 2025. PMID: 40906020 Free PMC article.
-
Mitochondrial Transfer as a Novel Therapeutic Approach in Disease Diagnosis and Treatment.Int J Mol Sci. 2023 May 16;24(10):8848. doi: 10.3390/ijms24108848. Int J Mol Sci. 2023. PMID: 37240194 Free PMC article. Review.
-
Ovariectomy and High Fat-Sugar-Salt Diet Induced Alzheimer's Disease/Vascular Dementia Features in Mice.Aging Dis. 2024 Oct 1;15(5):2284-2300. doi: 10.14336/AD.2024.03110. Aging Dis. 2024. PMID: 38913044 Free PMC article.
References
-
- Anil D.A., Aydin B.O., Yeliz D., Burcin T. Design, synthesis, biological evaluation and molecular docking studies of novel 1 H-1,2,3-Triazole derivatives as potent inhibitors of carbonic anhydrase, acetylcholinesteraseand aldose reductase. J. Mol. Struct. 2022;1257:132613. doi: 10.1016/j.molstruc.2022.132613. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

