Autophagy Inhibitor Chloroquine Downmodulates Hepatic Stellate Cell Activation and Liver Damage in Bile-Duct-Ligated Mice
- PMID: 37048098
- PMCID: PMC10092998
- DOI: 10.3390/cells12071025
Autophagy Inhibitor Chloroquine Downmodulates Hepatic Stellate Cell Activation and Liver Damage in Bile-Duct-Ligated Mice
Abstract
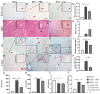
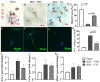
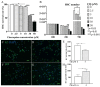
Hepatic stellate cell (HSC) activation via the autophagy pathway is a critical factor in liver fibrogenesis. This study tests the hypothesis that chloroquine (CQ) treatment can prevent autophagy and HSC activation in vitro and in vivo in bile-duct-ligated (BDL) mice. Sham-operated and BDL mice were treated with either PBS or CQ in two 60 mg/kg doses the day (D) before and after surgery. On day 2 (2D), HSCs were isolated, and their biological activities were evaluated by measuring intracellular lipid content, α-sma/collagen, and expression of autophagy lc3, sqstm1/p62 markers. The treatment efficacy on liver function was evaluated with serum albumin, transaminases (AST/ALT), and hepatic histology. Primary HSCs were treated in vitro for 24 h with CQ at 0, 2.5, 5, 10, 30, and 50 µM. Autophagy and HSC activation were assessed after 2D of treatment. CQ treatment improved serum AST/ALT, albumin, and bile duct proliferation in 2D BDL mice. This is associated with a suppression of HSC activation, shown by higher HSC lipid content and collagen I staining, along with the blockage of HSC autophagy indicated by an increase in p62 level and reduction in lc3 staining. CQ 5 µM inhibited autophagy in primary HSCs in vitro by increasing p62 and lc3 accumulation, thereby suppressing their in vitro activation. The autophagy inhibitor CQ reduced HSC activation in vitro and in vivo. CQ improved liver function and reduced liver injury in BDL mice.
Keywords: anti-fibrosis; autophagy; bile duct ligation; chloroquine; stellate cell transformation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Conway O., Akpinar H.A., Rogov V.V., Kirkin V. Selective autophagy receptors in neuronal health and disease. [(accessed on 19 January 2023)];J. Mol. Biol. 2020 432:2483–2509. doi: 10.1016/j.jmb.2019.10.013. Available online: https://www.sciencedirect.com/science/article/pii/S0022283619306096. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

