The Impact of Phase-Specific Macrophage Depletion on Intestinal Anastomotic Healing
- PMID: 37048112
- PMCID: PMC10093464
- DOI: 10.3390/cells12071039
The Impact of Phase-Specific Macrophage Depletion on Intestinal Anastomotic Healing
Abstract
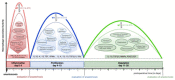
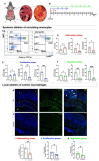
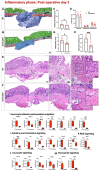
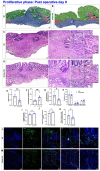
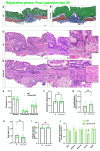
Intestinal anastomotic healing (AH) is critical in colorectal surgery, since disruptive AH leads to anastomotic leakage, a feared postoperative complication. Macrophages are innate immune cells and are instrumental in orchestrating intestinal wound healing, displaying a functional dichotomy as effectors of both tissue injury and repair. The aim of this study was to investigate the phase-specific function and plasticity of macrophages during intestinal AH. Transgenic CD11b diphtheria toxin receptor (CD11b-DTR) mice were used to deplete intestinal macrophages in a temporally controlled manner. Distal colonic end-to-end anastomoses were created in CD11b-DTR, and wild-type mice and macrophages were selectively depleted during either the inflammatory (day 0-3), proliferative (day 4-10), or reparative (day 11-20) phase of intestinal AH, respectively. For each time point, histological and functional analysis as well as gene set enrichment analysis (GSEA) of RNA-sequencing data were performed. Macrophage depletion during the inflammatory phase significantly reduced the associated inflammatory state without compromising microscopic AH. When intestinal macrophages were depleted during the proliferative phase, AH was improved, despite significantly reduced perianastomotic neoangiogenesis. Lastly, macrophages were depleted during the reparative phase and GSEA revealed macrophage-dependent pathways involved in collagen remodeling, cell proliferation, and extracellular matrix composition. However, AH remained comparable at this late timepoint. These results demonstrate that during intestinal AH, macrophages elicit phase-specific effects, and that therapeutic interventions must critically balance their dual and timely defined role.
Keywords: DTR; IBD; anastomotic healing; inflammation; intestine; macrophages; monocytes; mucosal inflammation; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Colonic anastomotic healing in the context of altered macrophage function and endotoxemia.Int J Colorectal Dis. 2011 Jun;26(6):737-46. doi: 10.1007/s00384-011-1171-2. Epub 2011 Mar 15. Int J Colorectal Dis. 2011. PMID: 21404056
-
Selective and specific macrophage ablation is detrimental to wound healing in mice.Am J Pathol. 2009 Dec;175(6):2454-62. doi: 10.2353/ajpath.2009.090248. Epub 2009 Oct 22. Am J Pathol. 2009. PMID: 19850888 Free PMC article.
-
Effects of macrophage depletion on characteristics of cervix remodeling and pregnancy in CD11b-dtr mice.Biol Reprod. 2019 May 1;100(5):1386-1394. doi: 10.1093/biolre/ioz002. Biol Reprod. 2019. PMID: 30629144 Free PMC article.
-
Roles of Macrophage Subtypes in Bowel Anastomotic Healing and Anastomotic Leakage.J Immunol Res. 2018 Feb 18;2018:6827237. doi: 10.1155/2018/6827237. eCollection 2018. J Immunol Res. 2018. PMID: 29670921 Free PMC article. Review.
-
Postoperative non-steroidal anti-inflammatory drugs and colorectal anastomotic leakage. NSAIDs and anastomotic leakage.Dan Med J. 2012 Mar;59(3):B4420. Dan Med J. 2012. PMID: 22381097 Review.
Cited by
-
In situ 3D bioprinted GDMA/Prussian blue nanozyme hydrogel with wet adhesion promotes macrophage phenotype modulation and intestinal defect repair.Mater Today Bio. 2025 Mar 5;31:101636. doi: 10.1016/j.mtbio.2025.101636. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40161927 Free PMC article.
-
The Influence of Apremilast-Induced Macrophage Polarization on Intestinal Wound Healing.J Clin Med. 2023 May 9;12(10):3359. doi: 10.3390/jcm12103359. J Clin Med. 2023. PMID: 37240465 Free PMC article.
-
Combining proteins with n-3 PUFAs (EPA + DHA) and their inflammation pro-resolution mediators for preservation of skeletal muscle mass.Crit Care. 2024 Feb 1;28(1):38. doi: 10.1186/s13054-024-04803-8. Crit Care. 2024. PMID: 38302945 Free PMC article. Review.
-
Layer-specific molecular signatures of colon anastomotic healing and leakage in mice.Mol Med. 2025 Apr 1;31(1):124. doi: 10.1186/s10020-025-01167-9. Mol Med. 2025. PMID: 40169980 Free PMC article.
References
-
- Bosmans J.W.A.M., Jongen A.C.H.M., Bouvy N.D., Derikx J.P.M. Colorectal anastomotic healing: Why the biological processes that lead to anastomotic leakage should be revealed prior to conducting intervention studies. BMC Gastroenterol. 2015;15:180. doi: 10.1186/s12876-015-0410-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

