Inhibition of LRRK2 Attenuates Depression-Related Symptoms in Mice with Moderate Traumatic Brain Injury
- PMID: 37048114
- PMCID: PMC10093681
- DOI: 10.3390/cells12071040
Inhibition of LRRK2 Attenuates Depression-Related Symptoms in Mice with Moderate Traumatic Brain Injury
Abstract
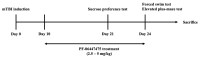
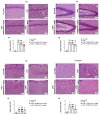
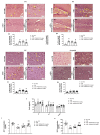
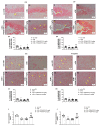
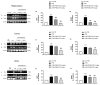
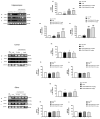
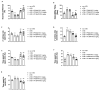
Moderate traumatic brain injury (mTBI) has been associated with emotional dysregulation such as loss of consciousness, post-traumatic amnesia and major depressive disorder. The gene Leucine-rich repeat kinase 2 (LRRK2) is involved in protein synthesis and degradation, apoptosis, inflammation and oxidative stress, processes that trigger mTBI. The aim of this study was to investigate the role of LRRK2 in reducing depression-related symptoms after mTBI and to determine whether inhibition of LRRK2 mediated by PF-06447475 could have antidepressant effects. Moderate traumatic brain injury was induced by controlled cortical impact (CCI) and mice were treated with PF-06447475 at doses of 1, 2.5 and 5 mg/kg once daily for 14 days. We performed histological, immunohistochemical and molecular analyses of brain tissue 24 days after mTBI. Furthermore, the tissue changes found in the hippocampus and amygdala confirmed the depression-like behavior. PF-treatment with 06447475 significantly reduced the histological damage and behavioral disturbances. Thus, this study has shown that mTBI induction promotes the development of depression-like behavioral changes. LRRK2 inhibition showed an antidepressant effect and restored the changes in the copper/glutamate/N-methyl-D-aspartic acid receptor (Cu/NMDAR) system.
Keywords: depression; moderate traumatic brain injury; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Hesperidin attenuates depression-related symptoms in mice with mild traumatic brain injury.Life Sci. 2018 Nov 15;213:198-205. doi: 10.1016/j.lfs.2018.10.040. Epub 2018 Oct 21. Life Sci. 2018. PMID: 30352242
-
Anxiolytic- and antidepressant-like effects of Silymarin compared to diazepam and fluoxetine in a mouse model of mild traumatic brain injury.Toxicol Appl Pharmacol. 2018 Jan 1;338:159-173. doi: 10.1016/j.taap.2017.11.012. Epub 2017 Nov 22. Toxicol Appl Pharmacol. 2018. PMID: 29175455
-
Serotonin 5-HT1A receptors modulate depression-related symptoms following mild traumatic brain injury in male adult mice.Metab Brain Dis. 2019 Apr;34(2):575-582. doi: 10.1007/s11011-018-0366-4. Epub 2019 Jan 3. Metab Brain Dis. 2019. PMID: 30607822
-
Pharmacotherapy Effectiveness in Treating Depression After Traumatic Brain Injury: A Meta-Analysis.J Neuropsychiatry Clin Neurosci. 2019 Summer;31(3):220-227. doi: 10.1176/appi.neuropsych.18070158. Epub 2019 Jan 14. J Neuropsychiatry Clin Neurosci. 2019. PMID: 30636565
-
Depression after mild traumatic brain injury: a review of current research.Neuropsychol Rev. 1998 Jun;8(2):95-108. doi: 10.1023/a:1025661200911. Neuropsychol Rev. 1998. PMID: 9658412 Review.
Cited by
-
Analysis of MicroRNA-Transcription Factors Co-Regulatory Network Linking Depression and Vitamin D Deficiency.Int J Mol Sci. 2024 Jan 17;25(2):1114. doi: 10.3390/ijms25021114. Int J Mol Sci. 2024. PMID: 38256187 Free PMC article.
-
Rebalance of mitophagy by inhibiting LRRK2 improves colon alterations in an MPTP in vivo model.iScience. 2024 Sep 16;27(10):110980. doi: 10.1016/j.isci.2024.110980. eCollection 2024 Oct 18. iScience. 2024. PMID: 39635134 Free PMC article.
-
The Inhibition of Prolyl Endopeptidase (PREP) by KYP-2047 Treatment to Reduce Myocardial Ischemia/Reperfusion Injury.Antioxidants (Basel). 2025 Apr 8;14(4):442. doi: 10.3390/antiox14040442. Antioxidants (Basel). 2025. PMID: 40298805 Free PMC article.
-
Astrocyte-mediated inflammatory responses in traumatic brain injury: mechanisms and potential interventions.Front Immunol. 2025 May 8;16:1584577. doi: 10.3389/fimmu.2025.1584577. eCollection 2025. Front Immunol. 2025. PMID: 40406119 Free PMC article. Review.
-
CK and LRRK2 Involvement in Neurodegenerative Diseases.Int J Mol Sci. 2024 Oct 30;25(21):11661. doi: 10.3390/ijms252111661. Int J Mol Sci. 2024. PMID: 39519213 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

