Rat Ovarian Function Is Impaired during Experimental Autoimmune Encephalomyelitis
- PMID: 37048118
- PMCID: PMC10093247
- DOI: 10.3390/cells12071045
Rat Ovarian Function Is Impaired during Experimental Autoimmune Encephalomyelitis
Abstract
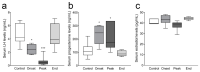
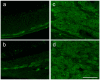
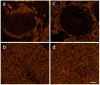
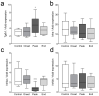
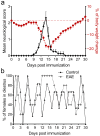
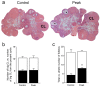
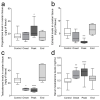
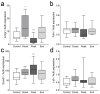
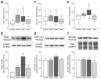
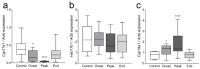
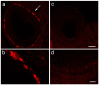
Multiple sclerosis (MS) is an autoimmune disease affecting the CNS and occurring far more prevalently in women than in men. In both MS and its animal models, sex hormones play important immunomodulatory roles. We have previously shown that experimental autoimmune encephalomyelitis (EAE) affects the hypothalamic-pituitary-gonadal axis in rats of both sexes and induces an arrest in the estrous cycle in females. To investigate the gonadal status in female rats with EAE, we explored ovarian morphometric parameters, circulating and intraovarian sex steroid levels, and the expression of steroidogenic machinery components in the ovarian tissue. A prolonged state of diestrus was recorded during the peak of EAE, with maintenance of the corpora lutea, elevated intraovarian progesterone levels, and increased gene and protein expression of StAR, similar to the state of pseudopregnancy. The decrease in CYP17A1 protein expression was followed by a decrease in ovarian testosterone and estradiol levels. On the contrary, serum testosterone levels were slightly increased. With unchanged serum estradiol levels, these results point at extra-gonadal sites of sex steroid biosynthesis and catabolism as important regulators of their circulating levels. Our study suggests alterations in the function of the female reproductive system during central autoimmunity and highlights the bidirectional relationships between hormonal status and EAE.
Keywords: EAE; multiple sclerosis; ovaries; progesterone; steroidogenesis; testosterone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Testicular steroidogenesis is suppressed during experimental autoimmune encephalomyelitis in rats.Sci Rep. 2021 Apr 26;11(1):8996. doi: 10.1038/s41598-021-88305-5. Sci Rep. 2021. PMID: 33903635 Free PMC article.
-
Sex-dimorphic changes in neuroactive steroid levels after chronic experimental autoimmune encephalomyelitis.J Neurochem. 2010 Aug;114(3):921-32. doi: 10.1111/j.1471-4159.2010.06825.x. Epub 2010 May 21. J Neurochem. 2010. PMID: 20492360
-
Dysregulation of the hypothalamic-pituitary-gonadal axis in experimental autoimmune encephalomyelitis and multiple sclerosis.J Neuroimmunol. 2003 Jul;140(1-2):78-87. doi: 10.1016/s0165-5728(03)00177-2. J Neuroimmunol. 2003. PMID: 12864974
-
Sex hormones in experimental autoimmune encephalomyelitis: implications for multiple sclerosis.Neuroscientist. 2001 Jun;7(3):258-70. doi: 10.1177/107385840100700310. Neuroscientist. 2001. PMID: 11499404 Review.
-
Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity.Stress. 2017 Sep;20(5):476-494. doi: 10.1080/10253890.2017.1369523. Epub 2017 Aug 31. Stress. 2017. PMID: 28859530 Free PMC article. Review.
Cited by
-
Alterations in the Hypothalamic-Pituitary-Adrenal Axis as a Response to Experimental Autoimmune Encephalomyelitis in Dark Agouti Rats of Both Sexes.Biomolecules. 2024 Aug 17;14(8):1020. doi: 10.3390/biom14081020. Biomolecules. 2024. PMID: 39199407 Free PMC article.
References
-
- Walton C., King R., Rechtman L., Kaye W., Leray E., Marrie R.A., Robertson N., La Rocca N., Uitdehaag B., van der Mei I., et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020;26:1816–1821. doi: 10.1177/1352458520970841. - DOI - PMC - PubMed
-
- Langer-Gould A., Smith J.B., Albers K.B., Xiang A.H., Wu J., Kerezsi E.H., McClearnen K., Gonzales E.G., Leimpeter A.D., Van Den Eeden S.K. Pregnancy-related relapses and breastfeeding in a contemporary multiple sclerosis cohort. Neurology. 2020;94:e1939–e1949. doi: 10.1212/WNL.0000000000009374. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

