Loss-of-Function Variants in DRD1 in Infantile Parkinsonism-Dystonia
- PMID: 37048120
- PMCID: PMC10093404
- DOI: 10.3390/cells12071046
Loss-of-Function Variants in DRD1 in Infantile Parkinsonism-Dystonia
Abstract
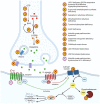
The human dopaminergic system is vital for a broad range of neurological processes, including the control of voluntary movement. Here we report a proband presenting with clinical features of dopamine deficiency: severe infantile parkinsonism-dystonia, characterised by frequent oculogyric crises, dysautonomia and global neurodevelopmental impairment. CSF neurotransmitter analysis was unexpectedly normal. Triome whole-genome sequencing revealed a homozygous variant (c.110C>A, (p.T37K)) in DRD1, encoding the most abundant dopamine receptor (D1) in the central nervous system, most highly expressed in the striatum. This variant was absent from gnomAD, with a CADD score of 27.5. Using an in vitro heterologous expression system, we determined that DRD1-T37K results in loss of protein function. Structure-function modelling studies predicted reduced substrate binding, which was confirmed in vitro. Exposure of mutant protein to the selective D1 agonist Chloro APB resulted in significantly reduced cyclic AMP levels. Numerous D1 agonists failed to rescue the cellular defect, reflected clinically in the patient, who had no benefit from dopaminergic therapy. Our study identifies DRD1 as a new disease-associated gene, suggesting a crucial role for the D1 receptor in motor control.
Keywords: DRD1; dopamine; dystonia; parkinsonism.
Conflict of interest statement
M.A.K. is a co-founder of Bloomsbury Therapeutics.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

