ABCB1 and ABCG2 Overexpression Mediates Resistance to the Phosphatidylinositol 3-Kinase Inhibitor HS-173 in Cancer Cell Lines
- PMID: 37048130
- PMCID: PMC10093605
- DOI: 10.3390/cells12071056
ABCB1 and ABCG2 Overexpression Mediates Resistance to the Phosphatidylinositol 3-Kinase Inhibitor HS-173 in Cancer Cell Lines
Abstract
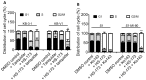
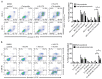
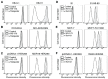
Constitutive activation of the phosphoinositide-3-kinase (PI3K)/Akt signaling pathway is crucial for tumor growth and progression. As such, this pathway has been an enticing target for drug discovery. Although HS-173 is a potent PI3K inhibitor that halts cancer cell proliferation via G2/M cell cycle arrest, the resistance mechanisms to HS-173 have not been investigated. In this study, we investigated the susceptibility of HS-173 to efflux mediated by the multidrug efflux transporters ABCB1 and ABCG2, which are two of the most well-known ATP-binding cassette (ABC) transporters associated with the development of cancer multidrug resistance (MDR). We found that the overexpression of ABCB1 or ABCG2 significantly reduced the efficacy of HS-173 in human cancer cells. Our data show that the intracellular accumulation of HS-173 was substantially reduced by ABCB1 and ABCG2, affecting G2/M arrest and apoptosis induced by HS-173. More importantly, the efficacy of HS-173 in multidrug-resistant cancer cells could be recovered by inhibiting the drug-efflux function of ABCB1 and ABCG2. Taken together, our study has demonstrated that HS-173 is a substrate for both ABCB1 and ABCG2, resulting in decreased intracellular concentration of this drug, which may have implications for its clinical use.
Keywords: ABCB1; ABCG2; HS-173; PI3K; multidrug resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

