Primary Cilium Identifies a Quiescent Cell Population in the Human Intestinal Crypt
- PMID: 37048132
- PMCID: PMC10093653
- DOI: 10.3390/cells12071059
Primary Cilium Identifies a Quiescent Cell Population in the Human Intestinal Crypt
Abstract
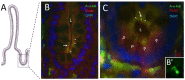
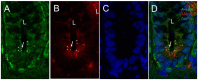
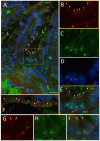
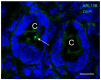
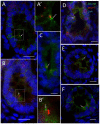
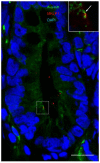
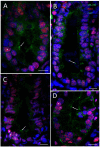
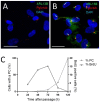
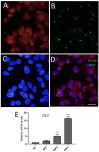
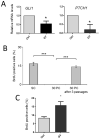
Primary cilia are sensory antennae located at the cell surface which mediate a variety of extracellular signals involved in development, tissue homeostasis, stem cells and cancer. Primary cilia are found in an extensive array of vertebrae cells but can only be generated when cells become quiescent. The small intestinal epithelium is a rapidly self-renewing tissue organized into a functional unit called the crypt-villus axis, containing progenitor and differentiated cells, respectively. Terminally differentiated villus cells are notoriously devoid of primary cilia. We sought to determine if intestinal crypts contain a quiescent cell population that could be identified by the presence of primary cilia. Here we show that primary cilia are detected in a subset of cells located deep in the crypts slightly above a Paneth cell population. Using a normal epithelial proliferative crypt cell model, we show that primary cilia assembly and activity correlate with a quiescent state. These results provide further evidence for the existence of a quiescent cell population in the human small intestine and suggest the potential for new modes of regulation in stem cell dynamics.
Keywords: BMI1; GLI; HIEC-6 cell line; Hedgehog pathway; intestinal epithelial cells; patched; primary cilium; stem cells; tubulin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
Analysis of the clonal architecture of the human small intestinal epithelium establishes a common stem cell for all lineages and reveals a mechanism for the fixation and spread of mutations.J Pathol. 2009 Mar;217(4):489-96. doi: 10.1002/path.2502. J Pathol. 2009. PMID: 19156773
-
Differential expression of the VLA family of integrins along the crypt-villus axis in the human small intestine.J Cell Sci. 1992 Jul;102 ( Pt 3):427-36. doi: 10.1242/jcs.102.3.427. J Cell Sci. 1992. PMID: 1506425
-
Expression of SV-40 T antigen in the small intestinal epithelium of transgenic mice results in proliferative changes in the crypt and reentry of villus-associated enterocytes into the cell cycle but has no apparent effect on cellular differentiation programs and does not cause neoplastic transformation.J Cell Biol. 1992 May;117(4):825-39. doi: 10.1083/jcb.117.4.825. J Cell Biol. 1992. PMID: 1349609 Free PMC article.
-
Human cell models to study small intestinal functions: recapitulation of the crypt-villus axis.Microsc Res Tech. 2000 May 15;49(4):394-406. doi: 10.1002/(SICI)1097-0029(20000515)49:4<394::AID-JEMT8>3.0.CO;2-K. Microsc Res Tech. 2000. PMID: 10820523 Review.
-
The gastrointestinal tract stem cell niche.Stem Cell Rev. 2006;2(3):203-12. doi: 10.1007/s12015-006-0048-1. Stem Cell Rev. 2006. PMID: 17625256 Review.
Cited by
-
Primary cilia and cancer: a tale of many faces.Oncogene. 2025 Jun;44(21):1551-1566. doi: 10.1038/s41388-025-03416-x. Epub 2025 Apr 29. Oncogene. 2025. PMID: 40301543 Free PMC article. Review.
-
An anaerobic in vitro flow model for studying interactions at the gastrointestinal host-microbe interface.NPJ Biofilms Microbiomes. 2025 Aug 11;11(1):160. doi: 10.1038/s41522-025-00800-z. NPJ Biofilms Microbiomes. 2025. PMID: 40789857 Free PMC article.
-
Gut aging: A wane from the normal to repercussion and gerotherapeutic strategies.Heliyon. 2024 Sep 12;10(19):e37883. doi: 10.1016/j.heliyon.2024.e37883. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39381110 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

