Ocular Delivery of Therapeutic Agents by Cell-Penetrating Peptides
- PMID: 37048144
- PMCID: PMC10093283
- DOI: 10.3390/cells12071071
Ocular Delivery of Therapeutic Agents by Cell-Penetrating Peptides
Abstract
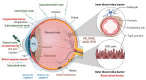
Cell-penetrating peptides (CPPs) are short peptides with the ability to translocate through the cell membrane to facilitate their cellular uptake. CPPs can be used as drug-delivery systems for molecules that are difficult to uptake. Ocular drug delivery is challenging due to the structural and physiological complexity of the eye. CPPs may be tailored to overcome this challenge, facilitating cellular uptake and delivery to the targeted area. Retinal diseases occur at the posterior pole of the eye; thus, intravitreal injections are needed to deliver drugs at an effective concentration in situ. However, frequent injections have risks of causing vision-threatening complications. Recent investigations have focused on developing long-acting drugs and drug delivery systems to reduce the frequency of injections. In fact, conjugation with CPP could deliver FDA-approved drugs to the back of the eye, as seen by topical application in animal models. This review summarizes recent advances in CPPs, protein/peptide-based drugs for eye diseases, and the use of CPPs for drug delivery based on systematic searches in PubMed and clinical trials. We highlight targeted therapies and explore the potential of CPPs and peptide-based drugs for eye diseases.
Keywords: CPP; drug delivery; ocular diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
An insight into cell-penetrating peptides: perspectives on design, optimization, and targeting in drug delivery systems.Pharm Dev Technol. 2025 Jun;30(5):521-547. doi: 10.1080/10837450.2025.2505000. Epub 2025 May 23. Pharm Dev Technol. 2025. PMID: 40356455 Review.
-
Anti-vascular endothelial growth factor biosimilars for neovascular age-related macular degeneration.Cochrane Database Syst Rev. 2024 Jun 3;6(6):CD015804. doi: 10.1002/14651858.CD015804.pub2. Cochrane Database Syst Rev. 2024. PMID: 38829176 Free PMC article.
-
Non-steroidal anti-inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery.Cochrane Database Syst Rev. 2017 Jul 3;7(7):CD010516. doi: 10.1002/14651858.CD010516.pub2. Cochrane Database Syst Rev. 2017. PMID: 28670710 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Advancements in Nanogels for Enhanced Ocular Drug Delivery: Cutting-Edge Strategies to Overcome Eye Barriers.Gels. 2023 Sep 4;9(9):718. doi: 10.3390/gels9090718. Gels. 2023. PMID: 37754399 Free PMC article. Review.
-
Photoreceptor-targeted extracellular vesicles-mediated delivery of Cul7 siRNA for retinal degeneration therapy.Theranostics. 2024 Aug 12;14(13):4916-4932. doi: 10.7150/thno.99484. eCollection 2024. Theranostics. 2024. PMID: 39267786 Free PMC article.
-
Cell-penetrating peptide-grafted AAV2 capsids for improved retinal delivery via intravitreal injection.Mol Ther Methods Clin Dev. 2025 Feb 3;33(1):101426. doi: 10.1016/j.omtm.2025.101426. eCollection 2025 Mar 13. Mol Ther Methods Clin Dev. 2025. PMID: 40027263 Free PMC article.
-
Enhancing DNA Vaccine Delivery Through Stearyl-Modified Cell-Penetrating Peptides: Improved Antigen Expression and Immune Response In Vitro and In Vivo.Vaccines (Basel). 2025 Jan 20;13(1):94. doi: 10.3390/vaccines13010094. Vaccines (Basel). 2025. PMID: 39852873 Free PMC article.
-
Exploring the Chemical Features and Biomedical Relevance of Cell-Penetrating Peptides.Int J Mol Sci. 2024 Dec 25;26(1):59. doi: 10.3390/ijms26010059. Int J Mol Sci. 2024. PMID: 39795918 Free PMC article. Review.
References
-
- World Report on Vision. Licence: CC BY-NC-SA 3.0 IGO. World Health Organization; Geneva, Switzerland: 2019.
-
- Common Eye Disorders and Diseases. CDC; Atlanta, GA, USA: 2022.
-
- Bande M.F., Mansilla R., Pata M.P., Fernandez M., Blanco-Teijeiro M.J., Pineiro A., Gomez-Ulla F. Intravitreal injections of anti-VEGF agents and antibiotic prophylaxis for endophthalmitis: A systematic review and meta-analysis. Sci. Rep. 2017;7:18088. doi: 10.1038/s41598-017-18412-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

